Prologue
Promoting growth is dangerous, especially if it is done in an un-natural way.
“Hormones and growth signals are tightly constrained, and highly optimized by evolution. Too little and the body atrophies over time, failing to renew muscle and nerve tissues. But too much and the body risks overstimulating some rogue cell, which may turn cancerous. Navigating between these two risks is a treacherous game, and the channel of safety is narrow. Eventually, the body falls to one side or the other, and so we must die.”*
I have read this narrative in different contexts and in countless variations. It is the central rationale of aging according to the mainstream of Western medicine. But we know it cannot be true. When we are infants, every endocrine growth signal is dialed up to the max, growth hormone is through the roof, cells are dividing like crazy, and yet cancer risk is very low. When we are old, growth hormone has dropped to nearly undetectable levels, cell division is lethargic, stem cells are few and less active–and yet the risk of cancer is at an all-time high.
Mainstream evolutionary theory says that the body is forced to make compromises, and this this is the ultimate reason for aging. The body doesn’t want to fall apart, but its first priority is to leave as many offspring as possible in the here and now, secondarily to preserve the body to continue to create offspring later on. Here-and-now is safer and also more effective, because of the earlier start generating grandchildren. So the body errs on the side of short-changing the infrastructure.
Why should the body have to compromise? The most popular and most standard theoretical answer is that its energy is limited. There just aren’t enough calories to do everything perfectly. This is the Disposable Soma theory of Tom Kirkwood, a beautiful theory that fails spectacularly when confronted with the real world. In theory, more energy should help the body avoid the need for compromise. We should live longer the more we eat. The truth is the opposite. In theory, spending energy on exercise should generate damage that needs repair, while consuming energy that could have been spent to protect from old age. Theory says that exercise should shorten life span, but the truth, again, is just the opposite.
Even if energy isn’t the limiting factor, it sounds so reasonable that the body should be forced to compromise because we so often encounter tradeoffs in different areas of our lives. Tradeoffs involve time and money, can’t be in two places at once, can’t have children and a career, must choose between two lovers who each fulfill parts of us. But it doesn’t always work this way. Computers become smaller and faster and cheaper and more energy efficient with each passing year. Filling our lives with love and fulfillment and a sense of gratitude and wellbeing also is the best single thing we can do to enhance our life expectancies. Sometimes you can have your cake and eat it, too; compromise isn’t always required.
So the question whether enforced compromises are implicated in aging must be answered by experiment and observation–it is not a matter of theory.
The root of the theoretical problem is the assumption that the body is doing its best to live as long as possible, and that aging and death represent failures of a system trying heroically to avoid them. But in this case, evolutionary theory leads us astray: the body is trying to kill it self on a schedule, as it is programmed to do.
The truth is that the body knows how to be young, and it knows how to be old. It does an exemplary job of both, each in turn. When the body is young, it is perfectly capable of growing, healing, producing offspring and repairing molecular damage, all accomplished simultaneously and without compromise. When it is old, it does all of these things imperfectly, if at all, as it gradually degrades and dismembers itself, using some of the same tools that were deployed for health and protection early in life: immunity, inflammation, apoptosis and cell senescence.
This view leaves open the possibility that medical science may find the body’s epigenetic clock, may learn how to talk to the body in its own language and fool it into thinking it is forever young.
So I am motivated to leave theory behind and look to the lab experiments for the answer: is it possible to boost growth and simultaneously to enhance longevity?
Creatine
Creatine is a very simple and common molecule with nitrogen and a COOH group like an amino acid. It occurs in all animal cells, more not plants. 1% of our blood is creatine. Biochemistry of creatine has been studied since 1832.

Creatine promotes creation of ATP, the cell’s short-term energy storage molecule. The way it works is this: ATP is adenosine triphosphate, and the 3 phosphates make it a high-energy molecule. In muscles and neurons that consume energy intensely, ATP is tapped, and one of the phosphates is degraded in the process, leaving ADP, or adenosine diphosphate. Creatine then steps in to recharge ADP back to ATP. It takes on a phosphate to become phosphocreatine, and then transfers the phosphate to ADP which is restored to its high-energy form, ATP. In times of rest, the process is reversed, as ATP gives up a phosphate to creatine, and an enzyme called creatine kinase generates phosphocreatine. Phosphocreatine can then serve as a short-term energy reservoir.
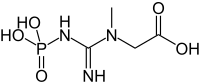
At any given time, there is something in the neighborhood of 100g creatine in our bodies. The amount varies widely. We make our own creatine in the kidneys and liver. But a substantial portion of our creatine is ingested, except that those of us who eat a plant-based diet get very little creatine. “Normal reference values for creatine are lower in vegetarians [ref]” We make less as we grow older, but it’s easy to lose the difference because of wide natural variation in creatine levels at all ages [ref].
Creatine was first discovered to improve athletic performance in 1912. Since stories emerged from the 1992 Olympics, creatine has been an increasingly popular supplement among body-builders. Creatine works especially well In combination with exercise, enhancing the benefit for strength and lean muscle mass. I found one study demonstrating these benefits in older men. I personally have been experimenting with creatine the past 5 months, and have noticed I can do more push-ups and chin-ups, and have gained a few pounds that I flatter myself to imagine are muscle. I have had a minor issue with cramping which might be a side-effect.
But it is only since 2010 that creatine has been known as an inhibitor of myostatin (aka GDF-8). Myostatin is a hormone that increases with age and degrades tissues, especially muscle tissues. Inhibiting myostatin leads to more strength and muscle mass, including a stronger heart. The action is not through more activity of muscle satellite (stem) cells, but of less wasting [ref].
Myostatin also promotes resting levels of growth hormone while suppressing spikes of growth hormone during exercise. This is generally thought to be a good thing, but the reasoning is indirect.
The best effect might be the increase in muscle satellite (stem) cells, but evidence is still thin [ref], and the effect may be temporary [ref]. There is limited evidence for creatine’s benefit to cognitive performance, especially in vegetarians and the elderly [another ref]. It has been mentioned in the context of treating Parkinson’s Disease. One study showed a decrease in the inflammation that comes after intense exercise.
The Bottom Line
100% of people in their 60’s and beyond develop sarcopenia=loss of muscle strength. No one likes it, and (if you need a clinical reason) sarcopenia increases risk of injury and very gradually closes the door to a world of benefits that derive from exercise. Exercise itself is the best way to slow sarcopenia, and creatine synergizes with exercise to help in maintaining muscle mass, strength and endurance.
Strengthening the heart is likely to be a good thing, and I have a belief that endurance and motivation and exercise and longevity are all so closely linked that I’m inclined to think there are ripple benefits from creatine. Some studies show that effects fade, so I’ll take it intermittently, one month on, a few months off. Drink much extra water while you’re taking creatine.
Long-term effects of creatine supplementation in humans have not been studied, except for one safety study that lasted a year and found no adverse side-effects and a small 4-year study that looked at a limited number of biomarkers.
You can purchase creatine as a powder, and it is not expensive. It is tasteless, and can be added to drinks (but not OJ), yoghurt, or smoothies. There is no consensus on dosage. I have seen recommendations ranging from 1 to 20 g per day.
————–
* Not a literal quote from anywhere, but I place this introduction in quotes just as a warning that I don’t believe it, and I don’t wish you to believe it.





