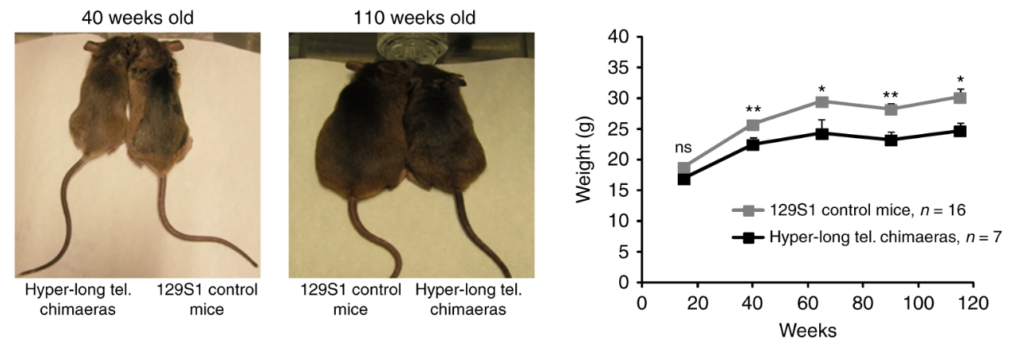
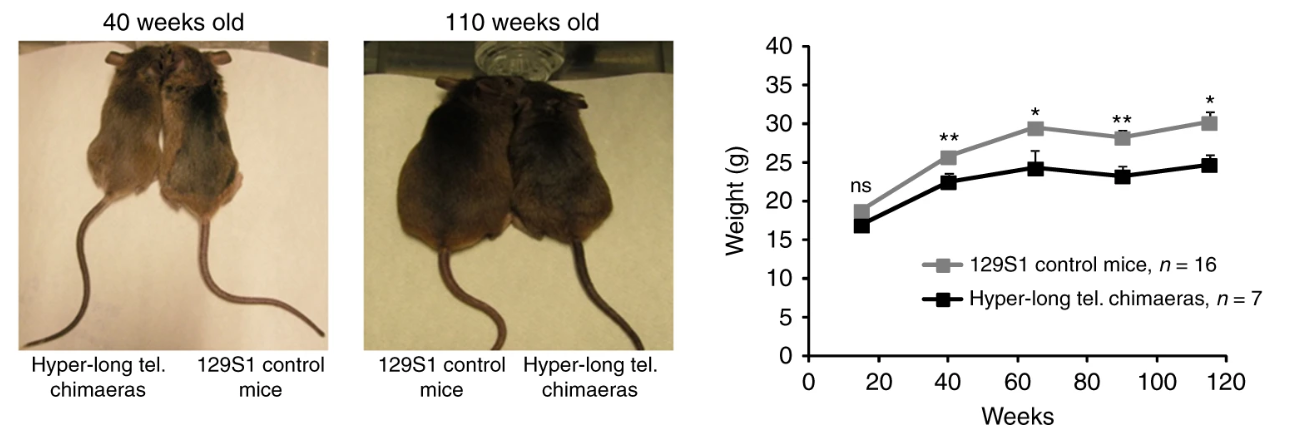
Mice have much longer telomeres than we do, long enough that telomeres never get critically short in a mouse lifetime. Yet, when designer mice were engineered to have even longer telomeres (hyper-long by any standard, longer than we can account for the use of them), these mice lived longer and were healthier in every way than mice with normal-long telomeres. Lab mice usually die of cancer, and these with the longer telomeres were protected from cancer, along with every other ailment that was looked at.
First, I ask your indulgence if I harp on the obvious: this result is not consistent with the prevailing theory of telomeres. In most vertebrates, telomerase is rationed so that telomeres are allowed gradually to shorten over a lifetime, and this is explained by most evolutionary biologists and geroscientists as an anti-cancer program. According to theory, in each species, telomere length has been optimized by natural selection as a compromise between longer telomeres (allowing stem cells to last longer without senescing) and shorter telomeres (which provide a firewall against cancer, a drop-dead signal when unchecked cell growth might be life-threatening). In contrast, experiments have frequently shown that longer telomeres lead to a lower cancer rate. Blasco’s new result is a clear case. We can’t explain telomere dynamics as a cancer prevention program.
(For background on what telomeres are and how they function, I refer you to my early blogs on the subject.)
But beyond this, there remain many mysteries. This study highlights the truth that we don’t understand the mechanisms. How exactly are hyper-long telomeres working on a biochemical level? What can a hyper-long telomere do that an extra-long (regular mouse) telomere can’t do?
Known mechanisms include:
- Senescent cells. Much of the literature has focused on the importance not of average TL but on the shortest because a few cells run out of telomere and become senescent, and they poison the rest of the body. This is called SASP, for Senescent-Associated Secretory Phenotype.
- Telomerase as an enzyme. Telomerase is best known for its ability to elongate telomeres, but there is evidence that it has other effects as well.
- TPE – the telomoere position effect. This is the only one that fits. Long telomeres wrap back around the end of the DNA, actually masking expression of the genes closet to the end of the chromosome. The Blasco study raises the possibility that we’re better off when the genes near the ends of the telomeres are silenced.
In my story, genes that have legitimate uses are turned against the body in old age. But there are no pure “aging genes” because it’s hard for such genes to evolve uphill (against individual selection). Has Blasco discovered an exception? Are these genes near the end of the chromosometrue “aging genes”? Or is it an example of evolved pleiotropy [my blog; BioRxiv preprint].
Context
Maria Blasco’s Madrid telomere lab has been at the forefront of this field for more than a decade. The new experiment is right on the bleeding edge of biotech and genetic manipulation, where the Blasco lab has staked out territory.
I learned that you can’t make mouse egg cells with long telomeres because the body’s process of making the egg standardizes the telomere length as it wipes clean the epigenetic markers and rewrites a starting imprint. How to get around this? Blasco grew eggs just until the third cell division (8 cells), then injected embryonic stem cells that had been grown saturated with telomerase to give them the hyper-long telomeres. Yes, this tiny embryo, just 8 cells in size, was micro-injected by hand with many stem cells, cloned to be genetically identical, so they would not fight immunologically with the cells already in the embryo. The injected cells were marked with a gene for green fluorescent protein (GFP) so descendants of the long-telomere stem cells could be identified later. The article doesn’t indicate exactly how, but the original 8 cells were induced to bow out, so that 100% of the cells in the mice that grew from these embryos had the GFP marker, and presumably, they all had the hyper-long telomeres as well. Thus, the lab made “designer” mice out of cells, every one of which had telomeres that (AFAWK) were longer than nature has any use for.
The stated inspiration for the experiment was to determine whether the hyper-long telomeres led to any detrimental effects. What they found was that hyper-long telomeres were beneficial in every way. The effect seems to be related to caloric restriction, since the mice are noticeably leaner and their insulin sensitivity remains high at advanced ages when mice usually become insulin resistant. Perhaps independent of these changes, the hyper-long mice had less DNA damage with age and more efficient mitochondrial metabolism.
Telomeres are full of surprises, and this may signal a new telomere mechanism, probably epigenetic, that is undescribed previously. But if it is to be described with known biochemistry, the only candidate is TPE, the telomere position effect. Long telomeres fold back on the end of the chromosome, masking some genes that are located near the end. It is already known that unmasking those genes when telomeres become short has pro-aging effects. But the new result involves telomeres that are (presumably) longer than anything that is found in nature or in the mouse evolutionary history. It follows that the hyper-long telomeres are folding back so as to mask genes that just happen to be near (but not to near) the chromosome end. In this picture, these genes just happen to be pro-obesity, or insulin-blocking. The effect is not evolved, but just a chance occurrence. I don’t like such explanations from chance, so I’d bet on a new telomere mechanism that is yet to be characterized.
Related study from the Blasco Lab
Another study (last summer) from the Blasco lab looked across species for relationships between telomere dynamics and species life span. This follows on the work of Seluanov and Gorbunova a few years ago. The previous work concluded that telomere length is most closely related to the body mass but not lifespan across rodent species. The authors tried to relate this to Peto’s Paradox, which is the observation that large, long-lived animals ought to have much higher cancer rates than observed, assuming that cancer results from a random transformation event in a single cell. In the new work, Blasco finds the closest correlation between lifespan and the rate of telomere loss.
We observed that mean telomere length at birth does not correlate with species life span since many short-lived species had very long telomeres, and longlived species had very short telomeres.
In short-lived species, telomere erosion happens much more rapidly: 7,000 base pairs per year are lost in mice, compared with less than 100 in humans.
In the old story [as I have reported it], telomeres shorten over a lifetime because stem cells lose a little telomere length with each cell replication. But this huge difference in telomere attrition rates can’t be accounted for in this way. Stem cells in mice don’t replicate 100 times faster than in humans. So something else is going on. Probably, there is partial expression of telomerase in a way that is programmed under control of natural selection. Telomere shortening with age has evolved in a way that contributes to aging via TPE. But (probably, by my account) telomere shortening is not the principal means of programmed aging, because the correlation between telomere length and age is too weak. Mike Fossel continues to promote the idea that relative but not absolute telomere length is a good indicator, and indeed a driver of aging. That sounds like it accords in the abstract with the new results, but details remain elusive.
The Bottom Line
It’s clear that telomere shortening plays a role in aging, though not a dominant role. It’s clear that telomere shortening is completely under the body’s control, therefore an evolved adaptation. Beyond this, the subject seems complicated, and there is good evidence that there are mechanisms involved beyond what we know about.
At a given age, telomere length in humans does not correlate with health risks. On this basis, I have argued that various methylation clocks are far better measures of biological age, and perhaps the GrimAge clock is best.
Discover more from Josh Mitteldorf
Subscribe to get the latest posts sent to your email.
When Olovnikov first made us realize that because of the inability of DNA polymerase to start a DNA chain de novo, it had to be the case that one strand at each end of a chromosome must shorten with every cell division. Thus we had a ‘programmed’ end to life as our telomeres shortened to the dysfunctional. However, that was then, and we know now that is not true and that telomere length is not a strict function of cell divisions, that it can be shortened by stress and lengthened by a number of different mechanisms including induction to pluripotency and somatic cell nuclear transfers. So I agree with Josh, that telomere attrition is but one of many mechanisms, both positive (chronic inflammation) and negative (turning off repair systems) that enforce lifespan limits.
Long telomeres and cancer:
Very easy explanation. Long telomeres slow aging. Only confusing to people who think cancer and aging are a different disease.
Well, I don’t think you have any evidence that cancer and aging have the same cause. Please do cite any reference. From evidence it doesn’t take much to convince me that cancer is a metabolic disease. Search for it if you’re interested.
All we really need to know is that rates of solid tumor cancers rise rapidly with age, at least up to age 90. Inflammation and insulin resistent seem to be strong indicators of cancer risk, and they both rise with age.
After age 90, cancer risk levels off and maybe it even declinces. The hormonal environment does not favor growth of any kind in old old age.
new paper from Blagosklonny “DNA- and telomere-damage does not limit lifespan: evidence from rapamycin”
https://www.aging-us.com/article/202674/pdf
“Failure of Rapamycin to extend lifespan in DNA repair mutant and telomerase-knockout mice, while extending lifespan in normal mice, indicates that neither DNA damage nor telomere shortening limits normal lifespan or causes normal aging”
Seems a bold statement on telomeres.
I added 4mg/weekly Rapamycin to my stack about a month ago.
I like a good Blagosklonny article. He is right right that ‘normal’ (lab) mice do not die from the DNA damage response (due to telomere shortening), hence rapamycin is beneficial to them. But that does not disprove the findings of the paper discussed in Josh’s article. In those findings mice born with (even) longer (than normal lab mice) telomeres were healthier and lived longer. So aging due to telomere shortening must not be (mainly) due to the DNA damage response (in lab mice). This is in accord with the gene expression changes found as telomeres shorten, long before replicative senescence.
The implied point made in the rapamycin study on mice with extremely short telomeres, which Blagosklonny criticises, is that rapamycin might not be beneficial for the very old (humans). Contrary to what Blagosklonny says on this point, mice with artificially short telomeres bred for multiple generations do seem to die of similar diseases to humans, suggesting that humans might suffer from replicative senescence in addition to gene expression changes from telomere shortening before that point. Therefore it probably wouldn’t be a good idea to give rapamycin to a very old, frail human.
We have known since the 1990s that telomerase is always on in rodent and lagomorph cells… the positive control in a TRAP assay is just ordinary rodent cells.
Even though I’m a telomerase enthusiast, it’s very weird that super-long telomeres make rodents live longer… they already have telomerase on anyway…
Agree that we don’t understand the mechanism yet. We DO know, and have since around 2003, that it isn’t the average telomere length in a cell that’s limiting… it’s the critically short telomere on one or a few chromosomes.
Always on? in all cells? Do telomeres not shorten with age in mice? I read elsewhere that many mice fibroblasts easily go immortalization, suggesting that at least some of the cells do not have sufficient telomerase activity for immortalization. If that happens some of the cells would turn senescent and potentially generate age related toxicity.
It might be always on in vitro, but it doesn’t stop telomere shortening in Vivo, as we know from numerous mouse studies that their telomeres shorten rapidly with age.
Yes, telomerase is always on in “mice”*, but mouse telomeres shorten rapidly.
*Remember that most papers are on lab “mice”, which are not mice… they have half the lifespan, bad cancer control, play too many video games etc.
Read Dr. Austead’s thoughts on “mice” and why his lab switched to real mice.
How does Liz Parrish’s latest blood work look? 😉
(If you try telomerase vectors at home, kids, make SURE you use inducible telomerase.)
My question or comment is related to a test to help determine if an aging intervention strategy worked.
My story is that I was diagnosed with leukemia, and spend two years undergoing chemo. Afterwards, it was obvious to me that the chemo had aged me 10 years. I used some of the ideas on this blog to try to do something about that. I think the dosages I used were a little high. My platelets went down to 4 (normal for me is 220). I almost died on several occasions, and I cost the Canadian medical system close to $100k.
It took 5 years, and my blood is finally (almost) back to normal. The question now is “did it work?”Did I reverse the 10 years of aging that chemo caused me? I tried to do a few flood tests when I was in Mexico to measure my glutathione levels and redox status. But, due to my bad Spanish, I was instead told that my kidneys were working just fine. Lol.
In any case, I think that realistically, if an aging clock said I was biologically 20 years old, I would be happy, but I wouldn’t really believe the results. The same is true if the test said I was 84. I would be sad, but I wouldn’t believe the test either.
In the end, the reason I know I had aged 10-15 years was because my recovery rate from exercise was terrible- it was what you would expect from someone in their early 60’s. I’m 42, and a fairly high level athlete.
I have very good data on my 10k running times right from kindergarten to now. Looking back, it’s easy to see a slow decay after I turned 27.
Nobody has ever been as fast a runner at 43 as they were at age 23. So, my test is simple. Once my blood recovers (which will probably take another 2 years), I’ll train for a 10k running race. We’ll just see how I do. If i’m as fast as I was in my 20’s, then this seems like accurate data to suggest that what I did worked. If I’m 15-20% slower then I was when I was 27, well, at least I beat cancer even if I didn’t manage to undue the long term effect of the chemo. Thoughts?
I’d say you dodged a bullet, and you have an opportunity to be grateful for the health most of us take for granted.
I think you’re right Chris. Real age reversal would increase exercise capacity. But improved exercise capacity doesn’t necessarily imply age reversal (although in your case we can probably discount this).
I’ve read that those who’ve undergone chemo have lots of DNA damaged bone marrow cells and this is the reason you get fragility in those who later put on weight – attempts to produce adipocytes lead to cellular senescence.
Just some thoughts, you might want to be careful if trying senolytics.
I would be curious of what an exosome IV might do. I have had one so far and feel really good but it is a slow process and subtle. You won’t feel anything immediately. A little pricey as well.
From all the puzzle pieces I have seen during my years of aging reserarch , the only explanation that makes sense is the telomere positioning effect….that is my immediate gut feeling…
So what does this mean from an evolutuionary perspective? My guess is that maxiumum lifespans of a species vary over time. Probably telomeres are the aging sytem of last resort. Take the mouse for example, for the most part telomeres do not affect aging that much when they contuinually shorten (for 3 generations) compared to the current evolved phenotype.
So mice are killed off by other aging systems operating independently of telomeres that kiick in before telomere shortneing does any damage.
Now allow the mouse to evolve a longer and longer liferspan until all the other aging systems are defeated. you are left with mice that die from telomere shortening once they have evolved lifepsans of say 20 years. So maybe the extra cancer causing genes that are covered up by the lab- lengthened telomeres are the last aging system that prevents mice from evolving life spans longer than 20 years. This aging system is active in current mice phenotypes in some cases where other agging systems have failed to kill them. And this last cancer-gene aging system will remain in place and active until the 20 year old mice can evolve even longer telomeres. We might be seeing this happen in naked mole rats who, live 30 years!! And they reportedly never get cancer. We need to look at their telomeric aging system to answer this question.
Here is an article that might be instructive>>
Telomeres and Longevity: A Cause or an Effect?
1 Department of Human Biology, University of Haifa, Haifa 3498838, Israel
Received: 2 May 2019; Accepted: 29 June 2019; Published: 1 July 2019
Abstract: Telomere dynamics have been found to be better predictors of survival and mortality
than chronological age. Telomeres, the caps that protect the end of linear chromosomes, are known
to shorten with age, inducing cell senescence and aging. Furthermore, differences in age-related
telomere attrition were established between short-lived and long-lived organisms. However, whether
telomere length is a “biological thermometer” that reflects the biological state at a certain point in
life or a biomarker that can influence biological conditions, delay senescence and promote longevity
is still an ongoing debate. We cross-sectionally tested telomere length in different tissues of two
long-lived (naked mole-rat and Spalax) and two short-lived (rat and mice) species to tease out this
enigma. While blood telomere length of the naked mole-rat (NMR) did not shorten with age but
rather showed a mild elongation, telomere length in three tissues tested in the Spalax declined with
age, just like in short-lived rodents. These findings in the NMR, suggest an age buffering mechanism,
while in Spalax tissues the shortening of the telomeres are in spite of its extreme longevity traits.
Therefore, using long-lived species as models for understanding the role of telomeres in longevity is
of great importance since they may encompass mechanisms that postpone aging.
Hi Jeff, Not sure what the conclusion would be here. We have two long lived species: In one the telomere length shortens (as in shorter lived species), and in the other they lengthen slightly. In the end, it seems to me that telomere length isn’t that important as long as the species in question can compensate in other ways. Though clearly short telomeres can serve as a biomarker of aging and/or stress when comparing different members of the same species.
I’ve heard naked mole rats exhibit negligible senescence, defying normal age related increases in mortality seen in other mammals.
Naked mole-rat mortality rates defy Gompertzian laws by not increasing with age, https://elifesciences.org/articles/31157
Hi, I send for its practical consequences this article that I received yesterday,
Milk Fat Intake and Telomere Length in U.S. Women and Men: The Role of the Milk Fat Fraction Larry A. Tucker, Brigham Young University, College of Life Sciences, https://www.hindawi.com/journals/omcl/2019/1574021/
While telomeres are interesting, just like all the other mechanisms of aging that have been discovered, in the end it doesn’t really explain much. Do mice with extra long telomeres live many times as long as those with shorter telomeres? No. The same applies to MTOR down regulation via caloric restriction and/or rapamycin. It delays aging but does nothing to address the aging mechanism itself.
I think the closest we have seen to reversing and/or stopping aging (putting aside the recent HGH results recently mentioned by Josh) are the well known parabiosis experiments mentioned by Harold Katcher in his 2013 paper, and seem to form the basis for his recent work developing a blood borne “elixir” than can truly stop and/or reverse aging. The experiments mentioned by Harold (younger mice joined to an older mice) demonstrate that something in the BLOOD SUPPLY is making older mice younger, and vice versa. Similar results occur when old tissues are transplanted in younger mice – the old tissue becomes young again. This seems to me is the real solution, and what scientists should be focusing on!
I don’t know what Harold Katcher is working up in his lab but maybe it has something to do with this work by the Conboys. Reduce TGF-b1 and increase oxytocin.
https://www.leafscience.org/brain-liver-and-muscle-rejuvenated-by-calibrating-aged-blood/
With the Conboy’s work in mind I am drinking more green tea and taking some Myrcetin -EGCG supplements to reduce TGF-b1.
http://ejbio.imedpub.com/natural-compounds-targeting-transforming-growth-factorin-silico-and-in-vitro-study.php?aid=17673
Make your own yogurt to increase oxytocin. Lactobacillus reuteri upp-regulates oxytocin.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3813596/
Thanks, this is very interesting, and exactly in line with what I was mentioning. I will review the links carefully.
Other blood factors have been identified recently that affects neurons.
At the Stanford University they compared serum from young mice to that of old ones.
They identified two proteins (THBS4 and SPARCL1) rich in young mice blood, which created more synapses compared to that of old mice. When tested on human neurons they saw the same increase of synapse formation and activity.
ref: https://www.pnas.org/content/116/25/12524
This is great news – thanks for the link.
Good point Armando… we’ve known for decades that tissues and organs are rejuvenated if transplanted into young people. That is a REALLY good hint about where we should be looking.
Girl Scouts should stop selling trans fats door-to-door and sell plasma instead!
Not sure that is true. I seem to recall reading that transplanted tissues in humans retain the epigenetic profile of their donor. I’ll check through my papers and see if I can find the reference.
Cheers
Well, I know people that have 30 year old pig valves in their heart, and you don’t see a lot of 30 year old pigs around 😉
I guess valves are mostly made up of nonproliferative tissue where paracrine/endocrine signalling causes the age related decline.
However serial transplant experiments have proven that proliferative tissue like bone marrow gets exhausted by the second or third transplant.
Have you got a ref for that Gabor? And does that mean bone marrow has sufficient proliferative capacity for 2-3 normal lifetimes?
I wonder if it more an issue of expansion ex vivo rather than once it’s implanted. Culturing adult stem cells outside the body whilst preventing differentiation is often poorly done. But proper in vitro culture conditions (see conditional reprogramming) can expand cells indefinitely without (further) loss of proliferative capability.
For example this one
Two phases of engraftment established by serial bone marrow transplantation in mice
1989
Has a nice introduction as well.
This seems like an easier and relatively safe way to extend Telomeres.
https://www.ncbi.nlm.nih.gov/pubmed/26214555
Increasing telomerase 100% is still a very small boost in humans. Interesting paper though, a lot of people take those drugs.
It’s more like 300%. Probably better than you can get from TA-65.
Mechanism is probably via ROCK inhibition and de-differentiation of endothelial cells to progenitors.
Here’s an interesting presentation by Jerry Shay that talks about telomere length, TERT location on the genome, and certain interactions. https://www.youtube.com/watch?v=iOApLQLtUBQ
My take is that telomeres are just what they are supposed to be: protective caps at the end of the chromosomes to prevent that chromosomes fuse.
Telomeres are probably hit hard with DNA damage thus accelerated telomere erosion in short lived species.
I find it amusing that evolution could have protected mice better with longer telomeres at negligible cost yet it didnt do it – doesnt this goes straight against Medawar?
Probably short living and genetically unstable organism are better at adopting to their violent and chaotic niche. They have no libraries and universities to teach coming generations – they only have their DNA to write The Annals Of The Rodents.
In mammals, there seems to be a strong inverse correlation of tel. length and lifespan. Also with increased body mass. Same for telomerase expression.
Michael D West brought this to my attention on his youtube channel. His latest video featured a short talk by Woody Wright, who sadly passed away recently, where he talked about these connections.
The paper discussed in that talk is “Comparative biology of mammalian telomeres: hypotheses on ancestral states”. Open access over at pubmed. It contains a great graphic with the known telomere biology of many mammalian species.
Large mammals seem to have short telomeres, repressed telomerase expression, a good telomere protection mechanism, cell arrest mechanisms and longer life spans. Some argue that.. why invest so much energy in growing big if you are not going to live long. They also appear to have either supposed or already observed enhanced tumor suppressor mechanisms, such as the large number of p53 gene copies in elephants.
Interestingly, the ancestral mammalian make-up seems to have been telomerase repression and short telomeres. So at some point in mammal evolution it may have been advantageous to change these traits.
I am still not sure which kind of advantage this could provide. Faster growth perhaps? by reduction apoptosis and by allowing terminally diff. cells to proliferate further? If this is the case it could stand to reason certain species which short average lifespans or high mortality levels in the young may benefit by growing as fast as possible.
I think ubiquitous telomerase expression in short lived mammals, may serve as a lifespan limiting adaptation by easing the possibility of developing cancer.
Josh should be all over this and I’m surprised thw article didn’t focus on this point, GaborB. If there really is NO disadvantage to the individual then the only remaining possibility is a disadvantage to the species.
Or we’re all totally wrong and there might be a species clock that erodes telomeres down through the generations as a tool of evolution.
After some googling i found that mice as a species are very diverse karyotypically. Chromosome fusions are extremely common, e.g. in mountainous regions of the Alps mice are stratified into subraces by chromosomal fusions. As if some chromosomes were binary switches. Warm climate – normal karyotype, cold climate – some chromosomes fused. So there might be an evolutionary advantage in somewhat unstable telomeres.
For chromosomal fusions to occur telomeres must be getting removed. But we are told mice never erode their telomeres away. There is an obvious contradiction here. A possible solution is that some smaller chromosomes with short arms probably have much shorter telomeres that do erode away with age, even in mice. So we are back to the ‘shorts’ being what is important, not the average.
This is also a nice mechanism for new (mouse) species evolution, as with a different karyotype, you effectively have a new species that can’t interbreed with the old karyoptype.
Centella asiatica – new record on activating telomerase
https://www.researchgate.net/publication/335367218_Discovery_of_potent_telomerase_activators_Unfolding_new_therapeutic_and_anti-aging_perspectives
Uow! A very interesting and cheap solution! I saw the chart comparing to TA-65 and others. I wonder if it would be even better than TAM-818. One thing the article doesn’t make clear.. If the Centella extract is such effective when ingested as is in cells in vitro. I would like see more research to get its potential.
TAM-818 was 16% of control in skin cells. Triterpenoids are 17,3% of the same control, but for leucocytes. TA65 gives 3,1 times in skin cells but only 2 times in leucocytes (as indicated in this study). So it depends on the cell type, so the results are not directly comparable (apples to oranges ;/). But 17,3 is 8% better than 16%…
Thank you!
“But 17,3 is 8% better than 16%…”
I didn’t understand this..
17,3 / 16 = 1,08125 *100 = 108,125% – the former is 8% greater than the latter.
Thanks, fascinating find. Now we need to measure the effect in humans instead of cell culture.
The beauty of Blasco’s latest study is that just the telomeres were longer, no more telomerase, no (direct) reduction in ROS. Yet the longer telomeres resulted in better mitochondrial health, better metabolic health, lower cancer incidence and longer life.
There is no confusion between human and mice telomeres. And there is no need for mice cells to divide 70x faster to lose telomeres at 70x the rate of human cells. Mice have much higher ROS than human cells and this accelarates telomere loss despite active telomerase in some tissues. There might also be other mechanisms.
Michael Fossel is right. It would be interesting to see what the lengths of these hyperlong telomeres fall to with increasing age. By death had they reached the same average (and percentage of ‘shorts’) that normal mice reach by the time of their death?
We need more granularity on this. Not many people realise that chromosomes are all different sizes and the length of their telomeres is correlated to this. So it is possible to predict what chromosomes get short telomeres first in both humans and mice.
Telomerase selectively locates to the shortest telomeres and lengthens them.
Dear Dr Walker,
Can you give me a reference to the statement of preferentially lengthening the shortest telomeres? I ask this as I have been taking a supposed telomere gene promoter for a while now.
Francis Gatt
I think Michael Fossel, and Bill Andrews may be on the right track regards telomerase. They say cells and tissues look rejuvenated, and the pattern of gene expression is returned to a youthful state, iirc.
I’ve heard a bit different, that in telomerase positive cells the gene expression pattern is returned to a youthful state with the exception of about 95~ genes. The anomaly could be due to having telomerase constantly active or some other reason. But it seems that if most gene expression is returned to a youthful state, the epigenetics, which I think regulate gene expression, must have been restored to a youthful state. The youthful appearance of tissues under the microscope merely confirms the cells have been made young again.
The problem in vitro, is that telomerase may be added via inserting the TERT gene in an unnatural location in the genome. In this case it’s impossible to say for sure what gene expression changes are caused by this rather than telomerase being turned on.
The real question is would say a skin cell with sufficient telomerase to never senesce through telomere shortening be able to maintain its epigenome indefinitely, i.e. always remain a working skin cell?
We’d have to see what other adaptations negligible senescence animals with positive telomerase have. But it is conceivable most cells would remain functional for long periods barring damage.
Josh, do you see a connection between Dr. Paul Hiehans “cell rejuvenation” and Dr. Khavinson’s Epitalon?
I think that telomeres have a auxiliary role in aging. Telomeres attrition leads to cell quiescence and with mTOR that impairs autophagy causes a flood of unused nutrients aka cellular junk
Very interesting experience from Blasco lab. Indeed longer than normal telomeres modified outstandingly the epigenetic pattern of the mice.
As Josh concluded: to attribute that by chance to the silencing of genes located near the telomeres (kind of capping effect) could be very remote from reality.
I have already pointed out that each cell DNA coiled double helix emitted and received vortices magnetic fields (also called scalar magnetic waves) which result in an hologram (3D field looking like the living being ) regulating the cell assembly (on the functional and structural levels)
As far as I know in the US the BIO-ELECTRIC effects of this field made hologram have been hopefully detected by the TUFT university Michael LEVIN lab experiences in case of METAMORPHOSIS and MORPHO-GENETIC body transformations.
In a first experiment in 2011 they found that the cells of a frog embryo displayed a 3D pattern of cells membrane polarizations which when observed in fluorescent light where a 3D picture of the FUTURE TADPOLE superposed on the actual material embryo. Clearly an electric phenomenon (migration of very finely modulated ions trough the cells membranes) they call bio-electric was at play.
Electrically speaking , the cells received the instructions and got the energy needed to transfert their ions trough their membranes with a finely tuned intensity. The coding system of this process is obviously analog. Indeed the pattern of VOLTAGE (electric field generated by membranes polarization) superposed on the embryo when visualized looks exactly like the future tadpole. The origin of this hologram point to the DNA ( wrapped down double helix) of the cells where all the recipes to produce a tadpole are registered on.
Then later on in 2012 the same lab found that if they reproduced the EYE blue-print from the pattern of cells membranes polarization on the tail of a tadpole a full eye
was produced where it dont belong! So this electrical polarization blue-print impose the differentiation and the epigenetic pattern of a eye to a group of in-vivo cells which otherwise would be skin cells.
Finally, around 2016 they found that if they cut a leg of a frog, simultaneously a cell membranes polarization pattern (signaling the wound) appear at the place of the cut legs BUT ALSO at the same position on the valid leg. In matter of electro-magnetic field speaking, an unbalance appear between the two legs where the missing leg tend to distort the whole body hologram (the removed part of the leg do not participate any more in the hologram generation) and the result is an instruction for the uncut leg to repair in order to reestablish the body symmetry.
That is due to the fact that In matter of morpho-genetic, the same hologram emitted by the DNA instruct a perfect symmetry in the body growth so limbs have the same length right and left.
LEVIN labs did not detect (nervous system, blood factors…) how the left side of the body had been informed simultaneously from the amputation of the right side leg. They postulate that an unknown mechanism is at play.
Above experiences indicate clearly that a stationary waves field (shaped as an analog of the real body) provoke voltage adjustments at carefully chosen cells membranes. As noticed by LEVIN experiments (the eye on the tadpole tail) this field INSTRUCT differentiation and genes expressions pattern (epigenetic) of cells in order for them to construct and maintain a living body accordingly to the DNA helix content..
To summarize now and come back to this Blasco experiment, the existence of an electro-magnetic instructions field generated by the DNA imply:
A resonance between the emitter and the receptor of such waves. This condition imply a steady length and shape of the wave generator. This waves generator is the double-helix of DNA wrapped around the histones with special folding proteins in way of the telomeres.
The telomeres have a particular way of wrapping themselves with special proteins and this spatial arrangement modify itself while the telomeres shorten.
The total length of double helix and its way of wrapping change in most tissues of the body while we age because of telomeres attrition .Experiments of LEVIN lab show that the pattern of bio-electric voltage is FINELY TUNED and so is the field of stationary wave which instruct it.
It is therefore a given that the epigenetic change at the same time that the hologram (of a young person with his specific) when telomeres shorten OR ELONGATE. The Blasco mice had to cope all their life with some electro-magnetic instructions not corresponding to a normal mature mice. Some of their organs where instructed like the one of fœtus I assume.
Fascinating. Are you saying telomere loss is effectively changing the length of an antenna?
The DNA is an emitter and a receptor of magnetic field vortices (alternatively as needed) in each cell. The coiled DNA double helix length and shape has a direct effect on the waves RESONANCE frequency and consequently for these longitudinal wave on their modulation (informations delivered between the emitter and the receptor).
Aging in respect of that model is caused mainly by the decay of the shape of the magnetic regulating hologram over our lifetime when our télomères shorten.
A mice born with extra-long telomeres will very likely have cells irradiating not an adult mice body regulating hologram but more the regulating hologram of an earlier stage of life of the same mice.
In the TUFT lab. experiences of morphogenesis (metamorphosis), what triggered the magnetic regulating hologram of the EMBRYO to transform itself suddenly into the hologram of a TADPOLE should be a change in the DNA wrapping (and length?) arrangement in the cells of the embryo.
Researchers have found that oddly enough FROG embryos have shorter télomères than adult frog . But obviously more researches must be done in this matter of frogs telomeres length to conclude about what could, by modifying the double-helix coiled shape, trigger the stationary electrical field shape changes in relation with the metamorphosis stages.
Can you provide a link to the TUFT Lab research?
https://ase.tufts.edu/biology/labs/levin/research/spatial.htm
https://www.youtube.com/watch?v=RjD1aLm4Thg&t=292s
https://www.sciencedirect.com/science/article/pii/S0958166917302690
https://www.sciencedirect.com/science/article/pii/S0079610719300926
It’s seems that discovery of a new mechanism of telomere lengthening in T cells (lymphocytes B giving snippets of telomere up to 3000 pair length to lymphocytes T) puts all the previous studies on blood telomere lengh and (lack of) correlation with age into dustbin.
https://www.lifespan.io/news/a-new-mechanism-of-telomere-lengthening-in-t-cells-discovered/
It’s seems that the shortest telomeres putting cells into senescence as early as late 30-ties/early 40-ties and lingering senescent cells multiplying in number about that age and producing TGF-beta which blocks telomerase it’s painfully and forefront life length limiting factor. As I recall TA65 used at early forties give spectacular results, but used at late 40-ties give no result. The body has dead’s man switch which switched on then fight telomerase very efficiently. If and when this mechanism is switched off, even weak telomerase enhancers have unexpectedly big effect on telomere length (and at the same time demethylation of the DNA and restoration of mitochondria). So yes activating telomerase lengthens life. No, it won’t help old people. Not much alone.
So it must be exosomes…
Exosomes seem to be very powerful. Hopefully translational research catches up with exosomes soon.
Previously I thought engineering existing cells in vivo back to a youthful state must be next to impossible but the more I read on exosomes the more I believe they will be the main delivery vehicles of choice for the antiaging agents, be them telomerases, demethylases, OSK factors, whatever.
Indeed it is exosomes. I summarised the findings of the paper a while ago,
Some key takeaways:
1. Chunks of telomeres are transferred from APCs to T Cells (APC= Antigen Presenting Cell not Armoured Personnel Carrier although this analogy functions well if you image telomeres to be the personnel being carried);
2. This enables greater telomere elongation than via telomerase (which T cells also intermittently enable) during expansion;
3. This allows 3 folder greater expansion of the T cell pool than without such transfer from APCs;
4. The burden of the shortest telomeres (<3K bp) in T cells is reduced 4 fold with such transfer enabled;
5. Senescence markers such as beta-galactose are also prevented;
6. The chunks of telomeres are cut off the APC telomeres after Shelterin proteins are downregulated;
7. Telomeres are released in exosomes from the APCs, with an average of 5K bp of telomere in each exosome;
8. They are then fused by endonucleases to the receiving T cells' telomeres;
9. The T cells benefit by an average of 3K bp extension each over 24 hours ;
10. Various antigen and T cell signalling is normally required;
But the authors find you can substitute these with a calcium ionophore (moves calcium into cells) antibiotic called ionomycin;
11. The APCs will then release telomere filled exosomes into the cell culture even in the absence of T cells;
12. Incidentally this mechanism is conserved between humans and mice and murine APCs were able to extend the telomeres of human T cells in culture ;
13. They validated this process occurs in vivo, using fluorescent telomeres in APCs injected into mice and verified these were taken up into the nuclei of antigen activated T cells also injected into the mice ;
14. The authors suggest that the telomere transfer process is required to limit the senescent signalling of T cells with very short telomeres, which telomerase mediated elongation (~100-200bp) is unable to to prevent;
15. The authors suggest using telomere vesicles to solve various problems with T cells and age; finally
16. It is not clear what other cells use this process, or whether there is some sort of trade off with the loss of telomeres in APC cells to assist the rapid expansion of T cells.
PS
17. this process is different to ALT (alternative lengthening of telomeres), which uses DNA synthesis and which was eliminated as a cause by the authors.
Do you have some sources regarding the fact the TA65 gives exceptional results at early 40 but meh results after that?
Thanks
No, it’s just my personal subjective feeling using it myself :<<
TA65 is made of astragalus roots and does very little for telomeres. There is a company that have human clinical study that shows lengthen of telomeres and decrease in cellular age. Certified Nutraceuticals sells Telos95. It is sold through http://www.longevitybynature.biz
Understanding what causes telomere longevity in melanoma will undoubtedly pave the way for the commercial promotion of products that claim to lengthen telomeres to reverse or prevent ageing.