Week 3 – continued review of The Vital Question by Nick Lane

Nick Lane takes a look at the evolution of life on earth with an eye to explaining large-scale patterns, from a perspective based on the energy metabolism. In the first week, we talked about the origin of life and the structue of the cell. In the second week, we looked at the differences between eukaryotes and what came before, and asked about mergers of widely differing species. In this third and final installment, I want to look at sex and death, and also to advocate for two important concepts that could broaden Lane’s perspective yet further.
Sex
Sex is the exchange of genetic material. It was invented long before the first eukaryote. Bacteria freely pass circular snippets of DNA called plasmids among themselves, with little regard to where they came from or what they are for. But in eukaryotes, sex became formalized, with exchange strictly limited to another of the same species (this is the definition of species), and it became compulsory, a prerequisite for reproduction in multi-celled species. Many plants and some animals are hermaphrodites, with both male and female in one individual. But most higher organisms have two separate sexes. Lane proposes to explain all these patterns based on the most fundamental observation: the mitochondria, having colonized the eukaryotic cell and brought with them their own DNA, have to remain healthy and work hormoniously with the host cell.
bacteria enjoy the benefits of sex (fluid chromosomes) along with the speed and simplicity of cloning. But they don’t fuse whole cells together, and they don’t have two sexes, and so they avoid many of the disadvantages of sex. They would seem to have the best of both worlds. So why did sex arise from lateral gene transfer in the earliest eukaryotes?
This is an (uncharacteristic, for Lane) understatement. For anyone who thinks in terms of the dominant paradigm of the 20th Century, evolution is all about individual competition and selfish genes. Plasmids, as selfish genes, make perfect sense. But the way that sex is implemented in eukaryotes makes no sense from the perspective of selfish gene theory. The most successful members of the community have combinations of genes that work better than anyone else’s. What incentive do they have to share genes with their competitors, bringing their fitness down and their competitors’ fitness up? And the biggest violation of selfish-gene logic is the “cost of males”. Hermaphrodites have twice the fitness compared to diecious sex (2 separate sexes).
The standard view is that this is a mystery, an isolated phenomenon that has yet to be reconciled with selfish gene theory. I prefer to think that diecious sex is an unequivocal refutation of selfish gene theory, that evolutionary theory must expand to embrace a notion of fitness more sophisticated than “every gene for itself”.
Origin of Sex
Eukaryotes were around for half a billion years as single-celled protists. Like bacteria and archaea before them, they were single cells, but the cells were 100,000 times larger and had a great deal of structure and mechanics that the prokaryotes didn’t have.
Lane says sex arose very early in the history of eukaryotes. He cites as evidence (1) that the long list of traits that all eukaryotes have in common (but that prokaryotes lack) could only have arisen in an inbreeding population; and (2) even the simplest eukaryotes today (giardia is the example that Lane cites) have the genes necessary for meiosis=cell mergers and gene exchange.
Cloning may produce identical copies, but ironically this ultimately drives divergence between populations as mutations accumulate. In contrast, sex pools traits in a population, forever mixing and matching, opposing divergence. The fact that eukaryotes share the same traits suggests that they arose in an interbreeding sexual population. This in turn implies that their population was small enough to interbreed.
In a diverse population sharing genes, it is possible for different lineages to evolve different features, and then these features come together in a single offspring when they mated.
An alternative hypothesis due to Margulis is that these diverse features were too different to have been encompassed in a single species (a single, interbreeding population). Rather, the the different features that came together in eukaryotes evolved separately and then the separate species combined in rare cell-merger events, a process she wrote about as “endosymbiosis”, or acquiring genomes.
(How different are these two pictures, really? We know that individuals with very different features must have shared genes; perhaps it is only a subtlety to ask whether these very different individuals were part of one wide-ranging inter-breding population, or of separate demes that might be called different species.)
Sex and Reproduction were Different Functions
In the one-celled eukaryotes, sex and reproduction were separate and unrelated functions. Reproduction occurred by mitosis, simple cell division, producing two clones. Sex occurs via conjugation, in which two individuals merge their cells, and merge the cell nuclei temporarily. Their chromosomes mix, and as each chromosome finds its opposite number, genes can cross over between the two chromosomes. When the merged cell comes apart, the two individuals that go their separate ways are no longer the same two individuals that came together an hour earlier. Instead, there are two new individuals, each a hybrid.
Could mitochondria have “agitated for sex”?
Lane sees the cellular invasion by mitochondria as the source of everything eukaryotic. Sex, as we have seen, is a particularly thorny problem—not just the mechanics, but the fact that (short-term) selective pressures should have been acting against it. But while sex would not be adaptive for the host cells (in the short run), it would have provided the only effective way for the mitochondrial “infection” to spread. There must have been a long transition period in which the mitochondria were not fully domesticated, and had their own ideas about what it means to be “adaptive”. After mitochondria learned to be endosymbionts, they would have trouble surviving outside the host cell, and trouble penetrating the cell walls of other cells, in order to spread from one host to another. So perhaps it was the genius of the mitochondria to induce some chemical change that would soften the host’s cell wall, and to promote behaviors that would seek other cells to merge with, giving the mitochondria a chance to spread.
My take: this hypothesis has the virtue of being “conservative” in the sense that it fits well within the predominant selfish gene paradigm. What could be more selfish than for the mitochondria to want to spread themselves? But at a slightly deeper level, the main thing that the selfish gene paradigm has going for it is that it is supposed to provide an explicit mechanism for natural selection, i.e., that the gene that makes the most copies of itself is the one that prevails. In this case, Lane’s hypothesis suffers for want of a mechanism how the mitochondria were able to take control of the cell’s behavior and override the interest of the genes in the nucleus for which sex was a liability.
Difference between plants and animals
Mitochondria reproduce within a cell so their DNA is copied many times for each one time that the nuclear DNA is copied. Furthermore, mitochondria exist in an environment of high-energy chemistry (ROS) that is a constant threat to the integrity of their DNA. So we expect high mutation rates in mitochondria, perhaps high enough to cause permanent damage and impaired performance. Somehow, in domesticating its mitochondrial guests, cells had to learn to culture the healthy ones and eliminate the damaged ones. Otherwise, mitochondria would gradually mutate and degrade over time.
This is a genuine conundrum, about which there are really no cogent ideas in the literature. If natural selection keeps populations healthy (and even improves them gradually) by filtering out the dysfunctional, where is the selection on mitochondria as they reproduce within a cell? Most dangerous of all would be the possibility of Darwinian competition within a cell among the different mitochondria. Some mitochondria might devote less of their metabolisms to serving the host cell and more to reproducing faster than their sisters. This could produce an evolutionary advantage within the cell for the slackers, the least useful mitochondria. Selfish evolution of mitochondria is an existential threat to the partnership between mitochondria and host.
Lane devotes a whole chapter to speculation about the resolution of this problem. We know that nature has managed to keep mitochondria healthy over billions of generations, in all surviving eukaryotes, but we weren’t around to watch how the mitochondria were tamed or convinced to submit to the hegemony of the cell nucleus. What we have to go on are surviving patterns that may bear the imprint of this ancient battle. The fact that mitochondria are inherited through the female line only is one piece of data. A difference in strategy between plants and animals may be another legacy of the battle: any cell of a plant’s meristem can grow into a seed that grows to a new plant, but in animals, the “germ line is segregated”, meaning that there is specialized reproductive tissue, protected from the earliest stage of embryonic development. Lane relates this difference to the fact that animals have a higher metabolic rate, with more mitochondria that are more active, thus a lower mitochondrial mutation rate. There may even be a connection to the reason that females lose their fertility earlier than men; the mitochondria become more highly mutated late in life, and it would be a risk to the offspring to launch them into life with a stock of mutated mitochondria. Males can afford to reproduce later in life because they don’t contribute mitochondria to the offspring.
Aging and death
Lane doesn’t have a lot to say about aging in this volume, but he does note that aging only really became an option once the germ line was segregated. Germ line cells need to have full capacity for regenerating everything (pluripotency) but cells of the soma have the luxury of specializing, and one option is to differentiate and grow once and for all, creating an organ that must last a lifetime (like a brain or heart).
In the end, Lane’s explanation of aging lands at a place very close to conventional theories based on tradeoffs. The somatic tissues of the body can’t be simultaneously good at everything, and they are specialized to their differentiated purpose, to the detriment of the ability of regenerate. Hence they are prone to wear out over a lifetime. I find this explanation less compelling than many of his other ventures, but this is probably inevitable, since evolution of aging is the area where my own thoughts are most highly developed.
Lane goes on to describe his own version of the mitochondrial free radical theory of aging, which is not an evolutionary theory. He elaborates why, despite the many well-known failures of this theory in its naive form, he nevertheless finds a core of truth in it.
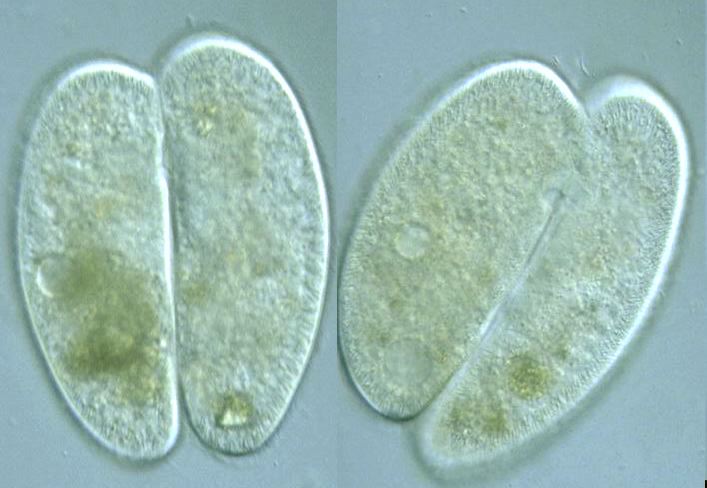
Sex and Death in Protists Presages Sex and Death in Multicelled Plants and Animals
The mechanics of conjugation in protists looks strikingly like the mechanics of sex in later multi-celled organisms. The way in which the cells merge, the crossover of chromosomes, the particular genes that are involved all point to a close relationship. Most striking is the strange mechanism of doubling the chromosome population before dividing it in half, and then in half again. The very arbitrariness of this behavior, and the fact that we see it both in protist conjugation and in male-female sex, is attests to the fact that latter evolved from the former.
I’ve said that sex and reproduction in one-celled eukaryotes are separate, unrelated functions. But there does exist one connection in ciliates, an advanced group of protists including the paramecium. Telomeres get shorter and shorter with each cell division. This is cellular senescence. It is permitted to continue, threatening the cell’s viability, because telomerase is repressed, and only comes out to restore the telomere when two individuals conjugate.
Thus, already in the early ciliates, cellular senescence has the purpose of enforcing conjugation. This ancient form of aging evolved to protect population diversity. And in higher organisms to this day, cellular senescence contributes to the death of the individual, assuring that the population continues to be enriched by new combinations of genes. The rationing of telomerase in protists presages the rationing of telomerase in you and me.
(William Clark tells this story in his very readable book, A Means to an End. My current project is a computer model demonstrating how telomerase rationing evolved on this basis.)

Where to go from here? Two suggestions
I am an enthusiastic supporter of Lane’s program, trying to understand the broad outlines of evolution, and why life is the way it is. I offer, from my own experience, two more themes that might complement his program.
- the conflict between what is adaptive for the individual and what is adaptive for the community, and how evolution has ways to suppress individual competition in order to create cohesive communities that are powerful competitors.
- adaptations at every level from chromosome structure to ecosystem structure that contribute to evolvability. It seems that natural selection has been a bootstrapping process, constantly increasing its own efficiency in the long term, even as it is selecting higher fitness in the short term.
Suppression of individual competition has been necessary for evolution to be able to find long-term solutions. This happens in somas that have the same genome as the germ line, and so their allegience to the germ line is not in question, and even in eusocial insects, where close kin selection helps to support division of labor in a functional community at a higher level of organization than the individual soma. David Sloan Wilson has devoted his career to the theory of multi-level selection, the ways in which natural selection operates simultaneously at the level of the individual and larger units of families, populations, and entire ecosystems. Often there are conflicts between what is good for the individual and what is good for the community, and the striking thing (taking the large perspective) is how consistently the communal interest has managed to take precedence, suppressing selfishness.


* * * * * * * * * * * * * * * * * * * * * * * * * * * * * *
Lane doesn’t mention “evolvability” by name, and tends to see it as random, chance event. “Adaptive” is the operant word, which signifies a Darwinian process,
changes to the genome itself, which might take the form of large deletions, duplications, transpositions or abrupt rewiring as a result of regulatory genes being inappropriately switched on or off. But such changes are not adaptive; like endosymbioses, they merely alter the starting point from which selection acts.
But I would suggest that there are too many of these properties of eukaryotic life that seem to serve not the gene or the individual carrying it, but the long-term viability of the community. We should expand our notion of a “Darwinian” process, if necessary, to accommodate the reality that evolvability has evolved. To be explicit: “Fitness” is the ability to survive and reproduce copiously and robustly. “Evolvability” is the ability to increase in fitness. Evolvability is the rate of change of fitness. We all agree that there is natural selection for fitness. The controversial idea is that there can also be natural selection for its rate of increase.
I have a personal relation to this idea. Harvard astrophysicist David Layzer wrote the first modern paper proposing the evolution of evolvability in 1980 when I was his student. Layzer’s analysis was ignored by the biology community for 16 years, until the time was ripe, and the same idea was re-cast into language more familiar to evolutionists by a prominent evolutionary theorist who teamed up with a creative and versatile computer scientist. Wagner and Altenberg generated a discussion that has developed and expanded to this day, but the revolutionary implications of this idea for evolutionary theory have yet to be assimilated. When the central importance of evolvability is fully appreciated, I predict that it will alter the foundations of evolutionary science.
Examples of evolvability adaptations include:
- Sex imposes a huge cost in individual fitness, but promotes evolvability. In fact, sex has benefits both for evolvability and for expanding the level of selection. As practiced by eukaryotes, sex gives each gene a stake in survival of the entire breeding community, and thereby promotes cooperation over selfishness [ref]
- Hierarchical signaling cascades, “command and control” with HOX genes controlling transcription factors and transcription factors controlling expression of many genes at once.
- Eukaryotic proteins are modular, with modules that are re-used in different combinations for different purposes. “Exons” are areas of the chromosome that code for pieces of protein.“Why do eukaryotes have genes in pieces? There are a few known benefits. Different proteins can be pieced together from the same gene by differential splicing…”
- Aging is an evolvability adaptation. This idea has a checkered history that goes back to August Weismann, but has recently been put on a firm mechanistic footing.
Though he never uses the word “evolvability”, Lane gets the message clearly about the benefit of sex, “fending off debilitating parasites, as well as adapting to changing environments, and maintaining necessary variation in a population.” In my view, he has yet to realize the profound implications of the fact the sex evolved for the sake of its contribution not to fitness but to evolvability. The fact that natural selection can favor not just fitness itself but also the rate at which fitness increases carries a deep message. “Evolvability” is not an individual trait of immediate value, but a property of an entire breeding community (a deme), spread through evolutionary time. The implication is that natural selection can enhance collective fitness, not just individual fitness, and that the long-term health of the community can be favored over the short-term advantage of the individual.
Evolvability is both a result and a cause of natural selection for traits (like aging) that benefit the community over the individual, even at a substantial cost to individual fitness. Evolution of evolvability is a bootstrap, a self-reinforcing process, a positive feedback system.
Sex in particular helps to elevate the level of selection from the individual to the community, because sex gives each gene a stake in survival of the entire breeding community, and thereby promotes cooperation over selfishness [ref].
This is a further clue, a connection between multilevel selection and evolvability.
Epilogue
I am full of admiration for Lane’s ambition to explain the broad properties of eukaryotic life, and he has made impressive progress pulling together diverse evidence into coherent theories. Lane is a biochemist and a “strict constructionist”, working within the predominant school of evolutionary theory, sometimes called the “New Synthesis” or “Population Genetics” or “neo-Darwinism”. My opinion is that to make further progress, he will find it necessary to venture beyond the neo-Darwinian framework to think about levels of selection, evolvability, and evolutionary ecology.
Discover more from Josh Mitteldorf
Subscribe to get the latest posts sent to your email.
Nice work, makes me want to read the book. I just wanted to say, with regards to mitochondrial ‘theories’ of aging is that a new publication (which Josh has access to from another forum) in Nature shows that there is mitochondrial dysfunction in the mitochondria of older cells, this does not result from either the mutation of mitochondrial genes, or the mutation of nuclear genes but it the result of a reversible epigenetic change that down-regulates two vital genes affecting mitochondrial processes.
And yes – paramecium is just as you say – it can undergo a number of cell divisions before it becomes replicatively senescent – but if it has sex (including sex with itself – autogamy) it resets its biological clock to zero and resets the number of cell divisions it can undergo before senescence. In the case of paramecium it appears that the mechanism of death by old age is the loss of function of many genes in its macronucleus – which can be restored by conjugation or autogamy – in which the DNA in the micronucleus is used to make a brand-new macronucleus (with fresh new genes), or the age can be reset to a non-zero, but much ‘younger’ state by eliciting DNA repair. In this case, the genes are reactivated, the paramecium becomes functional again and again capable of cell division – just (apparently) by having its DNA repaired (partly, at least).
I myself also wanted to start a simulation project a few christmas holidays earlier in which I would have tried to characterize the benefits of sex (crossovers) vs pure mutations. I wanted to go multidimensional, i.e. try if n side sex has any advantages over one or two side sex. Of course I never got to anywhere for lack of time.
However it could be such a nobrainer project, could be done by an undergraduate student anytime. I wonder what can be the main limitation with such kind of research?
My other project was machine code level GA on multicore processor farms. I made it work, but it could not find the trivial solution even on very simple problems like adding or multiplying two numbers, although it did converge a lot. Yes I know the increase in speed with machine code is linear and I can easily buy exponential performance gain in the cloud (Can I?). But the rigidness of machine code might just properly mirror the rigidness of protein structure, e.g. change in one triplet can ruin your whole protein likewise a bit change in machine code ruins your whole problem.
Another thing I would still do is to implement it for FPGA because the paralelism there more closely resembles real life than single threaded turing machines where most of GA is written. And crossover could be more meaningful in a modular FPGA than on a one thread CPU program. At least what I saw from my machine code project was that the effectively executed code essentially never grew over a few bytes as mutations destroyed diversity pretty soon.
Also I was thinking about keeping the genetic entropy high in my population but it was also an old research in the 80s and 90s and was not that successful. At least it did not become widespread or recognized.
Lately I was thinking about fuzzy search (exclude mutations that traverse the same path as the previous) but thats no longer GA.
Unfortunately I never had the time for these projects and I was always dubious if anything like new scientific wisdom could have been uncovered by them.
I wish you wouldn’t use a term like GA without defining it. However if I get the gist of what you are saying “genetic analysis” is what it represents? Anyway considering mendelian inheritence in that case may be limiting you to a view of heredity that is only gene-oriented and doesn’t consider such things as epigenetics (which is wide enough to encompass all forms of transgenerational communication – even symbolic communication I believe).
I’ve never worked with machine code but I know that anything that can be computed – any program at all – can be written in machine code – because it IS by the compiler. So there should be no restrictions except restrictions in thinking (and have to note a whole bunch of locations (same in assembly language code, but you don’t have to remember all the 0 and 1’s for each code word) – the fault lies not in the machine language but in ourselves – you want objects – start by giving their classes addresses. Easy for me to say though.
Anyway your greater point is exactly correct – make these computer models and try to get some information out of them – that’s the way it’s done in physics. However, make a mathematical model of a complex process you don’t really understand, necessarily leaving out variables you haven’t even considered and components you don’t know about, is not a model of reality – you can’t model what you don’t understand and don’t know (“let the computer figure it out” is something that only non-programmers could think)- it’s that simple, you can’t predict the actions of some actors when you don’t know all the actors or all their actions (in the case of the many pleiotropic enzyme/proteins found in mammalian cells). So until we understand the process – until we understand the simplest things like our own development from a single cell – our aging and death a continuation thereof, we won’t understand how to model the process. The old model – ‘wear and tear’ made such a good, easily computable simulations, and the current evolutionary model dependent on gene mutation and natural selection so easily lends itself to modeling (nothing too tough) – but what can you learn? The machine has no drive to survive, no originality – no ability to adapt ‘itself’ to its environment because, no self to adapt. So will gene duplication drive a gene to another purpose which will result in a new trait allowing it to survive better? How will it be directed, because there is considerable evidence that it will – and how will it be adopted and for what purpose (as though an computer-gutted ‘agent’ had any ‘purposes’ other than what you tell it explicitly – or how the organism might use a deformity such as a leg that turned into a fin as an advantage. How can you program that – what is the environment (as complex as the real environment of animals, internal and external, or a cartoon, or more likely a cartoon of a cartoon)- how complex is the ‘gene-product’ – how does the genotype relate to the phenotype – is it ever a simple relationship (rarely)?
So though I love computers and programming and robotics are my hobby (even though I have no time I’m working on something), but I don’t believe in the application of mathematics to a problem that is so poorly defined as present evolutionary theory.
You are right I was again sloppy in defining terms. I actually attempted doing Genetic Programing to create machine code programs by pure mutations and natural selection (procayotic life).
In this case the silicon chipin the simulation is the same as quantum physics of proteins and other organic polimers for life.
I was also interested in the very question brought up here – is there an evolvability advantage of sexual reproduction over pure mutation and copy. Can we prove it with computer models.
I believe the reason for computer models of evolution might not be good at capturing real world phenomenon because computers are sequential whereas life in a cell is massively parallel. Although I am quite sure you can model parallelism in sequential code, but you may lose too much performance. Thus my idea to do it in FPGA which is massively parallel. And there is a promising trend that FPGA resources would be integrated into cloud, so it could be more accessible. Currently you need expensive dedicated hardware to play with it.
But I agree that the main challenge as you state is to understand the cellular processes. thats much more worthy than creating an artificial world in silicon. And thats why I am a bit sceptical about interventions like telomerase elongation in vivo without thoroughly understanding the underlying processes. Telomerase activity might be epigenetically controlled in cells and should be switched on the right time in the right cells only to give us the benefitcal effects.
An unrelated question to Dr. Katcher. Since your publication about heterochronic plasma exchange in Biochemistry in 2013, has there been any advances on the field?
(I checked in google scholar, that article had only 3 citation including one from Josh. I am actually appalled that such groundbreaking research is so much neglected by the society. Also cant believe there is no ageing billionaire in the world who would finance such research.)
I too am appalled at the small number of citations – but I have faith that will grow. I’m more hopeful of the 2015 paper because it was published to the English-speaking world. We’re contacting aging billionaires, believe me.
I think that many ‘main-stream’ aging researchers disregarded that paper reflexively because I refused to genuflect to the doctrine of stochastic (random) aging – which everyone must (though you do have the choice of saying ‘antagonistic plieotropy’ instead of ‘wear and tear’ aging, or even call upon the doctrine of ‘disposable soma’).
I think I’m right and I (we) intend to prove it. So if anyone out there knows of some elderly billionaires who rather advance their own lives and the lives of millions, billions of people rather than taking that money to grave (to bribe the worms) would buy themselves years of additional youth – in any case – I as the procedure is safe (we’ve modified it a bit) and approved there’s not really a lot of risk, I think.
Drs Katcher and Mitteldorf… I appreciate the work you do because both of you focus on actual evidence. (I’ll be cross posting this review somewhere at LongeCity.org in a version that includes graphic figures sometime soon.)
Dr Katcher’s 2013 article (http://tinyurl.com/pstgs2u) transformed my thinking about Rejuvenation. Found the 2015 abstract (http://tinyurl.com/qxas2q2) and look forward to its publication.
But I’m puzzled that Dr Katcher’s 2013 Review doesn’t reference Chang’s 2007 demonstration of Rejuvenation via NF-kB Blockade and that their is a Primary Motif Map (http://tinyurl.com/mqjquf4).
By 2015, the evidence for NF-kB Transcription Activation as fundamental to aging In Humans has exploded as much as has the evidence for Telomerase Expression. There are at least 3 studies demonstrating a Longevity Benefit in Humans for NF-kB Transcription Inhibition via its Heart Rate Variability (HRV) surrogate marker. (See the 1st three graphic figures (http://tinyurl.com/k7skzyg). Meanwhile, has there been as clear a benefit demonstrated, In Humans, for longer Telomeres or HPE?
A question on my mind… Are there multiple Mechanisms of “exogenous control of age-phenotype at cellular and higher levels” within us? Or are the Rejuvenation/Longevity Benefits shown in recent studies merely different views of a single, larger Primary Mechanism that we do not yet understand.
I believe the latter is true and I provide evidence below for this view vis-a-vis NF-kB Inhibition and Telomerase Expression sub-Mechanisms.
1 – Evidence vis-a-vis Telomerase triggering supplements
— Astragaloside IV is a Telomerase Activator (http://tinyurl.com/qecphze) and it inhibits NF-kB (http://tinyurl.com/p9wcjk9).
— Astragalus inhibits NF-kB (http://tinyurl.com/oztgdpy)
— I like Bill Andrews and, in partnership with Isagenix, he is establishing a product to increase telomerase expression called Product B Isagenesis. It turns out that 90%+ of the 42 listed ingredients of Product B are implicated in at least 1 study as inhibiting NF-kB. See my Product B Ingredients google spreadsheet with NF-kB Inhibition Study Reference Links (http://tinyurl.com/ochm7ln).
— I find it curious that, in their respective 2007 and 2011 studies, Chang and DePinho used the same substance, 4-OHT, to blockade NF-kB expression and trigger telomerase expression (http://tinyurl.com/mqjquf4 and http://tinyurl.com/pvcudxf). Both report skin rejuvenation, but DePinho does not acknowledge Chang. Strange…
2 – Misc gene and supplement evidence about NF-kB Inhibition importance…
— FOX0 and SIRT1 inhibit NF-kB (http://tinyurl.com/q4cqyz7)
— Josh has written about Rapamycin. Turns out, it inhibits NF-kB (http://tinyurl.com/no3ovd8 and http://tinyurl.com/py75ey9)
— Metformin inhibits NF-kB (http://tinyurl.com/nkyxxhy)
3 – Evidence about Roles vis-a-vis p53 and Cellular Senescence
— p53 regulates cellular senescence and aging (http://tinyurl.com/qc7buw8).
— Short telomere length is a biomarker of cellular senescence (http://tinyurl.com/oor26bw).
— Telomerase expression is inhibited by p53 (http://tinyurl.com/oze4cca).
— NF-κB promotes senescence via control of the senescence-associated secretory phenotype (http://tinyurl.com/ogyqc87).
— NF-kB expression regulates p53 (http://tinyurl.com/o59r84h).
4 – Evidence about HRV, telomere length, telomerase, and NF-kB
Elissa Epel’s life work asks the questions that lead, inexorably, to the conclusion that the larger Longevity Benefit Mechanism underlying NF-kB Inhibition, measured by its HRV surrogate marker, is the same as that for Telomerase Expression. Her work demonstrates at least two things…
— Meditation, among other Techniques Promoting “Positive Cognition,” increases, both, HRV and telomerase expression. (http://tinyurl.com/pqsh6ae)
— Chronic Stress (i.e., Autonomic Ill Health, aka Lower HRV) shortens Telomeres (http://tinyurl.com/nrawp9w).
Meanwhile, Fredrickson has also found that Positive Emotions increase HRV (http://tinyurl.com/bxqwyym). And in another study, Fredrickson and Cole found that individuals with Eudaimonic Intent (aka, Noble Intent, a type of Positive Cognition (Emotion) that contrasts with Hedonic Intent) have reduced NF-kB expression in their peripheral blood (http://tinyurl.com/pzuupxu).
5 – Evidence about HRV and its inverse correlation with NF-kB Transcription Inhibition
— Establishing this evidence is basic physiology and biology research and has been the lifework of Karolinska Institute Honorary Doctorate Kevin Tracey, participant in 440+ studies per ResearchGate.
— Heart Rate Variability (HRV) is simultaneously increased with intracellular NF-kB Transcription Inhibition, especially in the Spleen, both via a Vagal-Acetylcholine mechanism (http://tinyurl.com/nogth6b).
— I recommend two additional study summaries to get a handle on the basic physiology/biology research that Tracey has spearheaded. (http://tinyurl.com/k7r7vzx and http://tinyurl.com/njy4kl6)
—————
The Longevity/Rejuvenation benefits of NF-kB Inhibition and Telomerase Expression are processes within a larger, single, primary mechanism. Dr Katcher aptly describes that mechanism as providing “exogenous control of age-phenotype at cellular and higher levels of biological organization.” (http://tinyurl.com/qxas2q2)
Sooner or later, those who believe otherwise will need to overcome the kinds of evidence presented above head on.
Cheers!
HDW, aka Steve Buss
Hi Desert Wizard – if you read my 2015 paper, ( http://www.ncbi.nlm.nih.gov/pubmed/?term=katcher-h), you’ll notice that I did quote the study you quoted and make a big point of the necessity for continuous nuclear control by NF-kB being required by tissue to maintain the aged -phenotype. So preventing NF-kB from entering the cells nucleus can rejuvenate mouse skin (and no doubt human skin as well). Yes I think that’s a major factor in aging.
So up-regulate Telomerase and downregulate NF-kb could be enough to reap big benefits? Could gene therapy deliver this payload to similar effect?
Also Harold lifespan.io is starting in August which is a crowd funding website devoted to longevity research, if you are struggling with funding you should apply with them. They are a 501 who do not take a chunk of the proceeds like indiegogo and allows your work to be in the public eye and supported. Millionaires are great if one can find them but if you have a small scale pilot study you could run meanwhile that may tempt further investment.
I can put you in touch with Oliver and Keith the CEOs if you like so we could get a pilot study funded for you? Might not be on the grand scale you are thinking but if you can conceive a way to do a scaled down pilot that proves efficacy that would break the ice and bring in the big money to go all out. Let me know if you want to explore that idea, you know where I am Dr Katcher.
We would like to begin ASAP Steven. If you can help us, put me in contact with folks we are ready.
great stuff, I mailed you a list of the basic prep they need and you already have costs done. I think we could totally nail a pilot study if we prepare for August launch.
Steve H – re: Downregulating NF-kB and Upregulation Of Telomerase… Frankly, after completing the research for that last post, I’ve lost track of what the difference is. If we are to believe Bill Andrews, Telomerase Activators are mostly also NF-kB Inhibitors, but a smaller percentage of NF-kB inhibitors are Telomerase Activators. Another point I didn’t mention… HMGB1 is important to understanding the NF-kB-Telomerase relationship…
Speaking of Rejuvenation… There was another 2012 study that many in the LE Movement have stopped talking about. And I mean the “The prolongation of the lifespan of rats by repeated oral administration of [60] fullerene” study (http://tinyurl.com/qb4futb). Four points to make…
1 – It has now been replicated with positive, but not as positive, result in a small animal study sponsored by LongeCity (http://tinyurl.com/qht6673).
2 – The ongoing, anecdotal, human, n = 1 self-experiments have mostly been very positive (http://tinyurl.com/mxjpjys).
3 – I believe the positive effects are mostly due to the same larger, primary mechanism of rejuvenation, referenced previously, that we don’t understand much about but which must at least include NF-kB Inhibition and increased Telomerase Expression as functions. The tell… Baati, et al, misconstrued the evidence of their own study about C60 accumulation in the spleen (http://tinyurl.com/p8zcyux). You’ll recall that Tracey is big on the importance NF-kB Inhibition in Splenic Macrophages (http://i.imgur.com/ojANTE8.png)
4 – Caveat Emptor… I am among the self-experimenters. I ingest C60-OO and apply it topically to my face. Improvements have occurred more frequently with dosage (frequency) increases. My facial skin is no doubt improved and goatee and mustache changed from mostly white-gray to salt and pepper. This change has been noticed by others without my prompting. I mix my own potion (http://tinyurl.com/oy8kr3b).
So, do I believe in the possibility of Rejuvenation In Humans? H*ll yes…
HDW
You might be interested to know bioviva has applied to longecity for project. Funding for a tert therapy pilot then.
I have mentioned nf kb as a possible target for down regulation and have been asked to forward papers for their team to Investigate.
Hey steve h… I’ve spent time reading about NF-kB and thinking about how to optimize its expression. Greater clarity about the NF-kB relationship to Telomerase Expression is a relatively new. I’d be pleased to assemble a list of the key summary literature titles that I think are important. I can be reached at the “High…” pseudonym I post under here @ gmail…
There is an angle on the NF-kB – Telomerase expression relationship that I don’t know much about… Where is the most potent Epigenetic Intervention point?
Thoughts?
Hi Josh,
have you ever considered the difference in aging between primitive animal lifeforms in Radiata – having only two germ layers and a radial body plan and diverse longevity and reproductive strategies- versus Bilateria – having three germ layers and two sided body plan and a strict lifespan and strict reproductive habits.
Seems like there are some very speculative clues linking gastrulation (only in Bilateria) to the epigenetic clock.
https://understandaging.blogspot.com/2016/10/p16ink4a-master.html
Also seems like immortalized epithelial cells that escape Ink4a/ARF related senescence go into a state that resembles epithelial mesemchymal transformation seen in gastrulazion (and wound healing).