Last week, a Danish study was published that tracked 65,000 people over a median of 7 years. The bottom line was that telomere length robustly predicts longevity, even after factoring out the effect of age, smoking, exercise, blood cholesterol, BMI, and alcohol consumption. This adds immensely to our knowledge of telomere length and its predictive power. For perspective, the original [2003] study by Cawthon detected the relationship between telomere length and mortality based on fewer than 200 subjects.
The new data set is large enough to show trends over all of the health-related lifestyle variables. Smoking, inactivity, weight (body mass index), and alcohol consumption all correlated negatively with telomere length. So it should not be surprising that blood pressure and LDL choloesterol also correlated negatively with telomere length, and it is then a foregone conclusion that mortality must correlate negatively with telomere length. This demonstrates without a doubt that unhealthy behaviors lead to shorter telomeres, as Epel and Blackburn have been telling us for a decade. (They have emphasized the converse: that healthy life choices lead to longer, healthier life through the medium of longer telomeres [Ref, Ref, Ref, Ref]).
The bottom line of the new, large study is the extra predictive power of telomere length, even after all these other lifestyle and indicator variables are factored out. Correcting for smoking, correcting for age, correcting for weight and cholesterol and exercise habits, there is still a powerful negative correlation between telomere length and mortality. The shorter your telomeres, the greater your chance of dying. The 10% of people with the shortest telomeres were dying at 1.4 the rate of the 10% with the longest telomeres, a result that was overwhelmingly statistically apparent (p<2×10-15).
|
Are Short Telomeres a Cause of Aging or Just a Marker of Aging? There are many traits associated with aging that are mere markers. For example, grey hair is associated with aging, but you don’t expect that coloring your hair will lead to longer life span. Even if you found a treatment that restored your hair color by rejuvenating the pigment in your follicles, you wouldn’t expect to live longer as a result. On the other hand, inflammation increases with age, and we know that it is not just a marker but a cause. Reducing inflammation leads to longer life. So are short telomeres like hair color or like inflammation? Can we reasonably expect that lengthening telomeres will lengthen life? Many lab scientists (and some gerontologists) think that it can’t be so easy to combat aging. Theory says that if telomerase could increase life span, then evolution would have granted us more telomerase. After all, the hTERT gene is already there in every cell, the metabolic cost of producing it is inconsequential. Telomerase is free, and it can be released with the turn of a metabolic switch. The theory continues: we know that diseases, lacerations, stresses all require more cell replication to repair them. This must leave telomeres shorter than they would be otherwise. Smoking and inflammation are also known to shorten telomeres. So (by this reasoning) people with shorter telomeres are expected to have shorter life expectancy because the shorter telomeres are telling a story that the person has suffered more stress. Short telomeres are only a symptom of aging, and not a cause. This new Danish study puts this theory to rest. At last there is enough data that corrections can be made for smoking, obesity, exercise, and all major life style variables that could conceivably be have an impact on mortality comparable to the large effect we find associated with telomere length. The correction is done using a statistical method called ANOVA, which can partition the causes of mortality into statistical bins and say how much is due to smoking, how much is due to blood cholesterol, how much is due to age, and how much is due to telomere shortening. Results from the study:
Conclusion: This demonstrates that age is the biggest factor in mortality, and telomere length has a strong effect, independent of age. All the health variables together are a small factor compared to age and telomere length. Short telomeres are not just a marker but a major cause of mortality. Evolution has turned telomerase off such that short telomeres substantially affect our life span. Turning telomerase on would not have cost anything, but that is not what evolution has done. So the theory is wrong that says evolution has already made our life spans as long as possible. Evolution has arranged for us to age and die “on purpose”. Withholding telomerase is part of an evolved death program. |
What does this say about the Cancer Hypothesis?
Suppose you believed that telomere length has been optimized by natural selection for a compromise between cancer prevention (short telomeres) and adequate capacity for tissue renewal (long telomeres). Then you would predict that, since the length is at an optimal level, there is a smooth, flat top in the mortality curve. People with slightly longer telomeres will have greater death rates from cancer, but lower from other causes; and people with shorter telomere length will have slightly greater death rates from other causes, but lower from cancer; and the sum (all-cause mortality) should be comparable for the two groups.
That would be the prediction. But what the Danish group found instead (consistent with other studies in the last 10 years, but now unassailable because of the large sample) is that all-cause mortality decreases with telomere length.
We can only conclude that telomere length is not optimized for maximal life span.
Caveats
The prediction is vindicated to the extent that telomere length is not as strongly associated with cancer mortality as it is with cardiovascular mortality. (Cawthon, too, found this in his tiny data set.) This shows that the theory is correct to the extent that short telomeres seem to offer some “protection” against cancer. But this “protection” is relative only to mortality from other sources. The net result of short telomeres is to increase cancer risk—just not as much of an increase as for heart disease. It is a safe bet that the reason for this increase is that short telomeres lead to more senescent cells (more inflammation) and less effective T-cells (less effective monitoring against early stages of cancer). Hence, the net result is that longer telomeres offer cancer protection that more than compensate the increased risk.
The one finding in the article that could most easily be mistaken for vindication of the cancer hypothesis is that there are three genetic markers of telomere length that were also tracked in these 65,000 subjects, and these markers are also correlated with higher cancer risk. The three genetic markers correlate with higher cancer risk and also with longer telomeres.
The way in which this result is reported is biased, a bit misleadingly, toward the standard cancer hypothesis. The authors write,
“We found that genetically short telomeres were associated with low cancer mortality but not low cardiovascular mortality, death from other causes, or all-cause mortality. This implies that genetically long telomeres are associated with higher cancer mortality.”
The misleading thing is the use of the words “genetically short telomeres”. You might read this and think it was referring to telomere length that the subjects were born with. But in fact, this was not measured. Without being able to go back in time, we have no way to know what was the subjects’ telomere length at birth. The fine print in the article tells us what they mean by “genetically long telomeres” is the variant of these three genetic polymorphisms that is statistically associated with longer telomeres late in life.
(A bit of background: a SNP is a “single-nucleotide polymorphism”. This refers to the smallest possible genetic difference. Our DNA is made of units labeled A, C, T and G, and the vast majority of your DNA is absolutely identical to mine and every other human beings’ DNA. What makes us unique is these small differences, and the lowest-level, smallest differences are these SNPs. A SNP is a place in the DNA where some people have an A while others have a C, for example, amidst a sea of letters before and after that are identical.
There are three SNPs in the region around the telomerase gene that presumably help to determine when and how much telomerase is transcribed. Some people make more and others less, based on these tiny differences. What the new study shows is that the version of these three SNPS associated with longer telomeres is also associated with higher cancer rates.)
This result is expected, and could hardly be otherwise. If the standard selfish-gene version of evolutionary theory works anywhere at all, it must work for the tinest variations. Indeed, that is where the theory logically must apply, and that is the only place it has been tested.
I have written extensively (in this blog here and here) that this same standard population genetic theory, the selfish gene, does not explain the big picture in evolution. By the big picture I mean sex, aging, cooperation, speciation, evolvability, the structure of the genome. But I would have to be radical indeed to deny that standard selfish gene theory can explain the small picture. For the record: I think that selfish gene theory offers a good account of SNPs.
Withholding telomerase, allowing cells and whole animals to senesce, is a “big picture” adaptation, built deeply into the structure of the genome. One small part of this picture is ruled by just three SNPs, and this small part appears to be guided by a tradeoff between cancer and other forms of mortality.
What else can be concluded from this huge new data sample?
This is far and away the world’s largest record of individual telomere lengths. Here is their scatter plot of telomere length vs age:
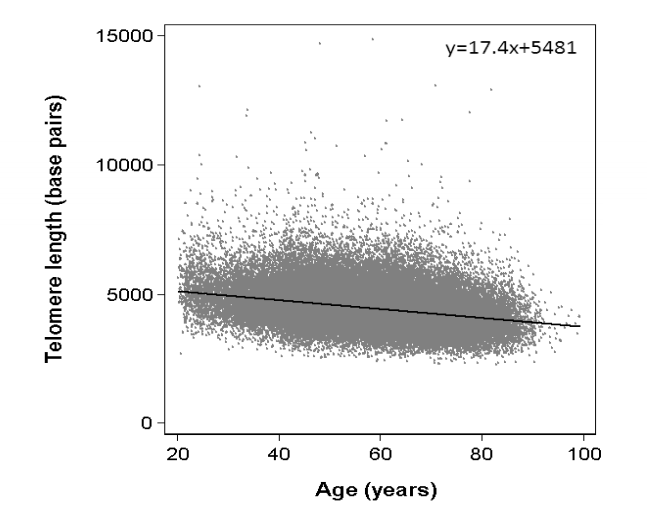
This is amazingly detailed compared to what had previously been available. Here’s an example of what we had been working with before the Danish study:

The first thing we notice is how many more data points there are in the new study. The second thing is that the scales are so different. In the older samle, between ages 20 and 80, telomere length decreases from 7,500 to 6,000 base. In the newer sample, telomere length decreases from 5,000 to 4,000 over a similar age range. This must reflect a difference in methodology for measuring average telomere length. Both studies characterize their target variable as the average telomere length for chromosomes in white blood cells from a blood sample. (Curiously, red blood cells have no cell nucleus, no chromosomes, no DNA. They are meant to do one thing only, to live a short time, and then be replaced.).
Both the new and old plots show a substantial scatter around the trend line. Standard deviation looks to be almost 1,000 bp, and there are especially many outliers thousands of bp to the north of the trendline. Although the downward trend is obvious with so many data points, it is also clear that it is not strong enough to account for the powerful mortality trend with age. For example, eyeballing from the plot, I would guess that ¼ of 60-year-olds have the telomere length of the median 90-year-old. But we know that the mortality risk of a 60-year-old is 17 times lower than for a 90-year-old (if Americans and Danes are similar), and if ¼ of all 60-year-olds fared as poorly as the average 90-year-old, then the difference between 60 and 90 could not be any larger than a factor of 4. This kind of reasoning reminds us that age is a larger factor in mortality risk, and independent of telomere length.
This suggests a strategy for seeking in the new data an answer to an important question for life extension science: How great are the potential benefits of telomerase activation? In the next few years, we expect telomere-lengthening treatments to be available, so that telomere length is no longer a factor in mortality. How many years can we expect this to add?
We might look for an answer in the factor 1.54 quoted above. People with the shortest telomeres have 1.54 times the mortality risk of people with the longest telomeres. Referring to the same life table I cited above, that factor of 1.54 represents five years of aging. From middle age onward, mortality is rising exponentially with a slope of about 0.038 per year.
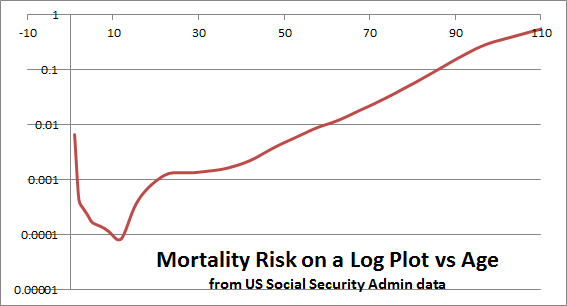
Only 5 years? From one perspective, 5 years is a huge benefit–about what we might get if there were a universal cure for cancer. But 5 years is a disappointing prospect for people (I am one) who have said that telomerase therapies are the most promising near-term technology for life extension. The calculation may be misleading for a number of reasons. For example, it may be that the average leukocyte telomere length is the most convenient thing to measure, but not the most salient. It may be that the shortest telomeres are more important than the average, and telomeres in stem cells are more important than telomeres in leukocytes. It may be that telomerase itself has benefits above and beyond the lengthening of telomeres [ref, ref]. Or it may be, as Mike Fossel has emphasized, that absolute telomere length is not as important as relative telomere length, separately calibrated not just for each species but each individual. For readers who know more than I about the biology of telomeres, I invite your comments.
Discover more from Josh Mitteldorf
Subscribe to get the latest posts sent to your email.
2 thoughts in my mind
1. This study further points to the disconnect between aging and telomeres. Yes, having short telomere is a health hazard, but not much cause for ageing. If one thinks about mice who age with long telomeres, this is not surprising.
Would mice age in a different ways than many other mammals? Hard to believe.
2. Maybe the study is vastly inaccurate. WBCs are derived frequently from stem cells. Their life span is much shorter than the average somatic cells. Stem cells and progenitors have telomerase activated.
A weighted average (or minimum) of all somatic cells should be calculated. How about the mice studies? Is telomere length measured across all somatic cells?
Interesting review article with inconclusive findings about the role of telomeres in aging.
http://biomedgerontology.oxfordjournals.org/content/66A/2/202.full
It confirms few of my concerns. TL measurements based on peripheral blood leukocytes are not concise measurements. Sometimes TL growths as time passes. Also there are doubts that leukocytes are a good proxy for other somatic cells.
Relationship between aging and telomere lengths not statistically significant many times.
My concerns about mice and telomeres also brought up there.
Maybe leukocyte telomere length is basically a measure of renewal potential of the active hematopoietic stem cell (or progenitor) population and not characteristic of the aging of the whole somatome.
Mice telomere mechanics are different to Humans so we cannot know for sure the effects until we try them in people in-vivo.
We do know that mTERT has rejuvenated every tissue type it has been tested on in-vivo. No increase in cancer was observed in those tests either.
I am not sure where Josh gets the only five years lifespan from? The graph shows mortality increases consistently with age and telomere loss so the two correlate to each other. True Telomeres may simply be a marker of stress etc… but I think they are far more important via the TPE effect on gene expression. We will not know unless we test this in a person though but if nothing else it should improve health via tissue regeneration. I would say health span naturally equates to lifespan anyway.
Interferon production is linked to DNA damage and Telomeres are also involved in this too.
http://www.impactaging.com/papers/v1/n7/full/100066.html
Interferon is shown to lead to senescence as shown in this recent study.
http://www.eurekalert.org/pub_releases/2015-04/uop-psi042315.php
To me removal of Senescent cells and telomere maintainence are good potential strategies for rejuvenation.
Yes, mice telomere dynamics different. But are mice aging differently from humans? I doubt it.
I somehow feel the emphasis on telomeres a bit of a dead end track. If you look at the graph you can easily find some% of 30 year olds has same leukocyte telomere length as some% of 80 year olds But we know immunosenescence is not existing at 30 but fairly widespread at 80 and bellow.
I also doubt that health span equals life span. Or it may be if we say tissue atrophy, immunosenescence etc are diseases.
If I disposed over huge sums of research money I would definitely follow either the rejuvenation blood factor track or the epigenetic clock of aging track.
I agree blood factors is a good idea, the parabiosis work has shown it rejuvenates and those factors in plasma also do.
The study I linked also shows factors can and do restore telomeres so you might be right and the best way to tackle aging is go for Blood factors and epigenetically control aging using them.
Unfortunately there are so many factors it could take decades to work out the mix, unless we use young plasma and can assay the factors quickly somehow.
I also meant improving health span is likely to equate to increased lifespan, if you are healthier you will likely live longer.
I still think Telomere elongation will lead to increased lifespan based on what I have read. Not the only cure for aging but should be significant.
We should hopefully find out shortly when its tested on localized skin in a person this year.
Josh, I think you’ve omitted to link to the study.
Thank you! I had the link in last week’s post, but forgot to include it today. Fixed.
This graph you show from Danish paper’s supplemental data (Supplemental Figure #2); I don’t understand it. What I don’t understand is how they come up with a formula of y = 17.4x + 5481. The x variable (age) should be *subtracting* from telomere length. Their regression says it is additive which makes no sense (or somehow I am looking at this very basic regression incorrectly). Any thoughts?
I didn’t notice that, but you’re right. There should be a minus sign in front of the 17.4.
Interesting new study from Salk institute on wrm protein would indicate heterochromatin disorganization as driver of human aging. Telemorese shortening maybe a secondary consequence?
Why would you think the causality runs in that direction rather than that short telomeres lead to heterochromatin disorganization?
Josh I agree with you on this one. Telomere shortening leads to genetic dysorganization. It appears that one critically short telomere ( 3-5 kb)may drive overall cellular pathways to induce senescence although those were animal studies. The telomere and sub telomeric regions are especially dense (closed chromatin) and generally hypomethylated suggesting they are epigenetically “protected”. As they shorten it definitely influences chromatin organization and methylation patterns although this area of study is in its infancy. Initially at least its likely that telomeres can influence both the chromatin makeup in their immediate vicinity and distant sites as well if the DDR (DNA damage response) is invoked by their shortening. Safest bet: it works both ways. But it is of interest that most of the “macro markers” of cellular aging- protein misfolding, glycosylation and mitochondrial biogenesis/mutagenesis/ox phos function improve when telomerase is added to aged cells. Again it could easily work both ways but clinically- who cares as long as we can reverse the damage!
Could you please drop a link on it? I cant find it in google scholar,
I mean, Scott Brown, could you please drop a link to the new study from Salk institute on wrm protein?
Best regards
GaborB
Josh
It seem to be implied in the article. What do you think of the findings? I felt it provides more evidence that aging is a less complex process than thought with likelihood for potential intervention.
This is another interesting study that can add meaningful information on this subject:
TELOMERE CHANGES PREDICT CANCER
“Changes in chromosomes years before cancer diagnosis could yield biomarker to predict cancer”
http://www.northwestern.edu/newscenter/stories/2015/04/telomere-changes-predict-cancer.html
hi,
I know that’s a bit ridiculous off-topic, since it’s really hard to believe there is anything beyond life. Indeed, there is nothing measurable that points to that. But many people believe in that, and it’s interesting to see how some effort is being made to make near death experiences as more than hallucinations by real scientifics.
I read the book of Eben Alexander because he’s got about 100 peer-reviewed paper, which is a way to say he’s very well scientifically formed and clever. He swears for his experience.
http://www.ebenalexander.com/
What do you think?.
Best. Santiago
Santiago,
The hard question of consciousness or why we are self aware has been bandied about for millenia by philosophers and today neuroscientists have joined in the fray. The bottom line is, does the brain create consciousness or not? If it does then your thoughts about the potential for afterlife would be correct. Your school of thought says we die and then there is nothing. However,if consciousness is not produced by the brain you would be wrong. Not only would there be an afterlife but somehow there may have been a before life experience. There is a lot of information about the controversy. Youtube has some interesting videos on the subject.
Stan
A couple of specifics: QPCR is a great and cheap way to look at average telomere length. It has uses in this exact kind of study where the “n” is in the thousands. But it tells nothing about %short telomeres or telomere dynamics with enough accuracy to predict anything about individuals ( or with regards to the cancer study- what is going to happen). Using average telomere lengths is of not much value when you do not have the other data. The other data is 1) generated by a more expensive test pioneered by Dr Blasco in Spain-e.g. not Menlo Park so it is not paid much attention to. 2) Cawthowne is from the original group of PCR scientists that includes the recent Nobel laureates so they all do only QPCR so where to most scientists look? to QPCR- the precedent is set this way. Using epidemiologic data to plot what happens to an individual is inherently dangerous when the test used to generate the data is not accurate or repeatable enough for the individual. I did the commercial QPCR test using my own blood labeled as mine and someone else’s of the same age. Same time same arm same blood draw. It varied by several hundred base pairs. The HTQFISH was almost identical. Sneaky me but it helped me answer the question of accuracy and repeatability for a lay person who would be doing this test and banking on the results.
Based on what I have seen and done in this field I do not think QPCR will be of use in the Individual prediction of anything.
Next to the epigenetic study linking specific histone and MSC markers to aging. Anything that impairs cell cycle regulation or DNA copying will accelerate typical senescence provided the cell cycle check points are intact (p53 etc). The fact that there is WRN dysregulation in the small group of “normally aged” people is probably the most important thing that study found. But in these people as opposed to the iPSC and MSC cell lines the finding was NOT independent of telomere loss (at least there was not comment to this effect in the paper whereas they specifically commented on this for the cell lines) so suggesting that the mechanisms are independent of telomere attrition is not a supported conclusion. By the same token the epigenetic disorganization stops cellular replication and inhibits one of the major drivers of telomere loss- cell division. ESC and MSC may have ways to replicate in vitro that are not found in vivo. Any stem cell scientist worth their salt will tell you there is a very big difference between behavior in culture and in vivo and that the later is far from defined.
It is of course possible that that there are “multiple entry points” to accelerate and thus decelerate aging. Telomeres are certainly one of them.
The mouse studies also bear comment. Mice do age like humans via telomere attrition.Again Blasco did these studies. They just age a lot faster even though their telomeres are usually at least 3X longer. But the percentage of short telomeres is predictive (again HT QFISH is needed for this information) Mouse metabolism is 7X that of human beings and mice do not appear to have the robust anti-oxidant defenses humans have. While direct free radical damage has been questioned as a “real” aging phenomenon it certainly appears this is the case for mice along with what appears to be driving free radical damage in humans: mitochondrial dysfunction, OxPhos uncoupling, and the related epigenetic re programming of the genome from repair and control of inflammation to the sloppy dysregulated chronic ongoing inflammation and even oncogenesis we see in older humans. As we learn more about the SAPS ( carol Greider)previously called senstatic activation by Mike West we will most likely see that aging is a self fulfilling inflammatory process and telomeres of course with their G-G-G make up are extremely sensitive to Ox damage.
Overall the studies (Blasco and DePinho) that show de aging in mice and life extension in mice may be of questionable relationship to humans but the mouse model of aging is far more robust than most of the uses for mouse testing especially cancer xenographs etc. I think 5 year life extension is low balling it Josh but I did not do the math!
Finally the real markers of biologic age will likely be stem cell telomere length. I am part of a study that is looking at just that in bone marrow, fat and PMC’s. We are not looking at dental sources because most of us can part with some blood marrow and fat, but are less inclined to sacrifice dental pulp or gingiva!
Fred – Wow! This is great information, and I’m very happy to have it on my blog page. Thanks so much…
– Josh
Thank you for writing it Josh and your interest in what I think is one of the most exciting fields out there. It is good to see scientists who are not made totally myopic by their fields looking into other areas of interest. Gives me hope that someday collaboration will speed up the answers we’d all like to see.
I wanted to clarify this statement “ESC and MSC may have ways to replicate in vitro that are not found in vivo.” What I meant was there may be individual pathways available to control cell cycle regulation in cultured cells that are not a major part of in vivo mechanisms. Cell division is cell division- how we get there and control it has potentially many entry and exit checks and balances. The very fact that just about every gene involved in this ( and DNA repair!) winds up being an oncogene is testimony at least in my mind lol!
Since you are interested in this science you might want to look at pub med under Jao Passos who I think is at Newcastle U and also Ron DePinho late of Dana Farber, and now MD Anderson. Both have done some interesting papers on how mitochondrial biology interelates with telomere biology- worth reading and ties a lot of loose ends together.
Finally my friend Mike Fossel has a new book due out in October. As the first person who stood up and said “It’s telomeres!” it is sure to be a must read. Thanks again -Fred
HI Josh yet again a few more comments based on questions from your readership and some comments on the blog. I hope you will indulge me.
1) Glad you pointed out the difference between “genetically longer telomeres” in semantics. The phrase longer telomeres is often used in misleading fashion. Everyone should keep in mind that commercially available telomere testing has only been around for a few years and is not exactly popular because up until now ( and most presumably long after now) most people including scientists have been saying “so what” to testing telomere length in the general population! University based telomere testing has only been around for a little over a decade for human use and as i pointed out its been QPCR for the most part and has some serious issues. Taken together this means the following: almost NO ONE has longitudinal telomere measurements for more than a few years. And certainly there are no large scale infant studies in the works as far as I know in order to establish a long term base.
2) Also most studies that report “longer telomeres” are guilty of the same problem: they do not have more than one measurement. They look at an activity such as exercise in one cohort versus another. The exercise cohort has longer telomeres and the impression is they”grew” their telomeres with exercise. Not so, they merely slowed the loss versus the non active group. Very good yes but not a life extender, You are looking at something that as Len Hayflick put it, ” Helps you approach the wild type age of the species”. Almost no study reports 2 measurements and all those that do use the most inaccurate method QPCR even with tiny studies (Ornish Blackburn on meditation and prostate ca as an example). No data on telomere dynamics,short telomeres and no histograms. For the record spot “real time” TRAP telomerase assays are far less useful than actual telomere measurements when deciding interventional outcomes.
3) Also on the “so what” side telomere measurement companies have had a serious up hill battle because no one wanted to believe what Mike Fossel said, ” It’s telomeres stupid!”. You would think the “n” of 65,000 would help but I betcha it doesn’t silence most of the critics who still want to believe its “just a biomarker”. Cal Harley’s Telome Health appears to be defunct, TeloME the salivary test thankfully disintegrated, and Life Length is struggling because their technology seems to be ahead of its time and is not QPCR. One would hope this study changes all that but I doubt it. Until there is a drug available ( like statins and cholesterol) people will discount everything else including nutracueticals/supplements.
4) Question from your readers- does PMC (white blood cell) telomere length correlate to other tissues,
Answer: sorta kinda! There are now at least 4 tissues where the correlation has been established: skeletal muscle, liver, small intestine, and skin. Note that all these are tissues that have inherent telomerase activation already ( and most likely are highly stem cell dependent for renewal)* and relatively short regeneration times with the exception of skeletal muscle. The absolute numbers vary by as much as 1000 base pairs with skeletal muscle cells having the longest telomeres ( and the lowest native telomerase activation- something we have seen in other tissues as they approach the post mitotic state- the less likely the regeneration, the more degree of telomerase activation). The telomere dynamics however- how relatively fast or slow the attrition happens and to what degree- are almost straight line mimics above or below each other when plotted on the same graph. This suggests a “global rate” and global correlation of aging in the body with a major caution. The major caution is, if the individual does something extreme and repetitive to a part of their body, that part will reflect increased damage. Example: pro basket ball players, wrestlers and Olympic lifters all have shorter than average telomeres in their chondrocytes and muscles but at least average telomere lengths in their PMC’s. This suggest it is possible to accelerate aging differentially by certain activities just as it may be possible to slow it down towards your “wild type” level by engaging in others such as diet sensible exercise and sleep. Bottom line: it does appear that PMC’s measurements are useful and valid to predict both health span and life span. On the other side of the coin it does appear to be possible to accelerate aging globally if one damages part of the immune system. Chronic CMV, HIV and cirrhosis ( alcoholic or now the sadly more common fat accumulation induced) all seem to lower PMC telomere length as well as liver and lymphoid tissue. My theory this is MSC induced since these are the progenitors of most of the immune system. Without too much detail, you have a nice dove tail if you look at senstatic activation,(SAPS) the sloppy inflammatory responses of the aged, Omega 6 induced over inflammation from diet and immuno senescence concepts and aging. Personally I believe the immune system is going to be the key much longer life and health span. First you have beat heart disease which is pretty easy for the normal genome: eat a lot less, eat fewer bolus meals, and rebalance your omega6/3 ratios and you will most likely hit your wild type risk of heart disease which should be 1/10 what it is now ( W. E. Lands “Fish omega 3 and Human Health). That would also reduce most of the other diseases of aging as well apparently. Then we need to beat the old man’s friend- infection and cancer which is where I personally think telomerase activation comes in,
Finally as to the SNP issue. Thank you for pointing that out to your readers. I would like to add the following thoughts: Chromosome 5 where hTERT resides along with the putative repressor of telomerase and more specifically the region of hTERT has been found to be “hypermutatable” in GWAS studies ( genome wide associated studies). More simply it is more likely for a mutation to occur there than many other places in the genome. Similar regions have been found in the APO region known for its association with heart disease and Alzheimer’s presumably via the gene products of this area and their intimate relationship with cholesterol. It may seem like an odd way to put it but the likelihood of cancer in hTERT mutants- at least one of them-being associated with increased cancer seems to be far outweighed by longevity. Gil Atzmon’s study in Ashkenazi Jews with the mutation showed significantly longer life span and health span so this may be the trade off.
Again referencing Jerry Shay’s immortal fetal fibroblast study in 1999. Constituitive telomerase activation seems only to be a problem if the genome is “unhealthy” as it already is say in cancer patients. Age is of course the major risk factor for non germ line cancer (which are at least 95% of all cancers) so SNP’s may not be at all the culprit. They simply may turn on telomerase enough to allow cellular replication for longer (longer life) but not enough to prevent eventual genomic instability and check point escape which in my opinion starts at the intersection of p53 and the mitochondria (the primary retrograde epigenetic drivers ala Warburg. Seyfried etc) not in the nucleus. Also Blasco’s AVV9 study increased telomerase up to 10 fold with decrement over a year. Net result no statistical increase in cancer even with life extension in both middle aged and old mice. So degree of activation seems to be the key. Better said “non constituitive” activation seems to be the key which makes one thing pharmacologic/neutracuetical activation of telomerase (TA-65)* is the most appealing way to do it. It also argues against those of us who do not have SNPs being too concerned about using other non constitutive ways to turn on telomerase.
Hope this intrigues you as much as it intrigues me.
Fred
* I had an interesting series of conversations with the authors of the Immortality Edge about this.
Hi fred,
thanks a lot for your comments, I think you have answered some of my questions.
Two more questions:
1. I did not quite get what you wrote on the tradeoff between longevity and cancer. Can you please help me understand
2. What do you think on the aging of non dividing cells, e.g. neurons, muscle cells. Is it related to the immune systems.
+1 what do you think about the parabiosis studies?
Gabor please be aware that the answers to your questions are by no means agreed upon and truth be told my opinions on these topics while educated are considered “radical” and speculative.
With regards to cancer versus longevity I was referring to the issue of SNPs that are associated with higher levels of hTert. In some cases this leads to longer telomeres and in that small group of individuals with the specific 3 SNPs present there was a higher incidence of cancer. The internet translated this into “Longer Telomeres Lead to Cancer”. As Josh pointed out for various reasons this is rubbish. My comment was simply that no one has studied these people longitudinally with TRAP assays to define how much extra hTert was actually produced, or with serial HTQFISH assays to evaluate their telomere lengths and dynamics over time. Since they are not immortal we can safely say they are not making enough telomerase to resist the aging process which still continues at some rate and experiencing the same genomic disintegration we all seem to have happen. For the record I am not saying I think telomerase alone will make people immortal. Since aging continues in these people it is not unreasonable to assume that whatever process one believes causes cancer for instance issues with DNA polymerase and DDR fidelity, mutations will still occur that could cause cancer. If the people live longer there will be more chance for this to happen. In point of fact looking at super centenarians we do not see specific longevity genes (sorry gang FOXO and sirtuins are included in my statement) and clinically we see simply a delay in the issues of aging until later. They still get heart disease, cancer Alzheimer Disease etc it just happens much later. The recent autopsy on the 115 year old Dutch woman opens up the possibility that stem cell exhaustion may be the culprit in the very old- again in the immune compartment specifically. Using telomere length for biologic age the rest of her body was “80” while here immune compartment had only 2 functional stem cells for clonal expansion. The very high mutation loads you would expect in someone this old were not found to be a factor at all. The short telomere length of her stem cells was the causative factor of her immunosenescence.
That is a nice transition to question 2 and yes I do think the post mitotic tissues like heart and brain do indeed experience immune dysfunction and inflammation related to telomere attrition. This I think is the basis for AAV therapies of Blasco Fossel and BioViva if I understand them correctly- microglia will be a main target. That said people tend to forget the simple fact that post mitotic tissues rely on highly mitotic tissue specifically the intact endothelium of the blood vessels that supply those tissues to be health and intact. Vascular endothelium is highly mitotic and telomerase dependent as well
Finally I am no expert on parabiosis but I assume you mean the Wagers studies and GDF 11. Very interesting and I am looking forward to seeing what else develops here. I would also personally like to see what happens in mitochondrial transfer experiments between young and old cells. If the results of the cancer experiments done with this design are any indication we might have yet another way to combat aging.
I am going to step aside from this blog for a bit so others can contribute and steer the direction of the discussions. Best, Fred
Fred what do you think about this study which showed Telomere length increased when exposed to hormones?
http://www.bloodjournal.org/content/114/11/2236?sso-checked=true
Whilst you say you are not an expert in Parabiosis and Plasma exchange I think the two are interelated, Telomeres influence gene expression and factors can influence Telomere length. I would be interested in your opinion of the study linked as so few studies measured changes to Telomeres when youthful factors are introduced. I think it makes a very strong case that Telomere restoration would yield significant results.
Very interesting but supplements are not going to be effective enough nor reach all cells to activate telomerase. Ta65 and other products are weak and simply not going to do the job as the liver simply breaks them down. I put such supplements into the snake oil category personally, gene therapy or very powerful drug intervention on htert is the only way to meaningfully upregulate telomerase.
I definitely agree with Fred that Stem cell Telomeres are likely the most accurate biomarker for age. Stem cells are really what telomerase therapies should be targeting rather than daughter cells, if anything it would be preferable to increase the length of telomeres in stem and progenitor cells so they continue to produce new fresh cells and allow the daughter cells to age and die and be replaced to reduce mutation/dysfunction in general cells.
A therapy to clear out the cells that refuse to die would be the ideal partner therapy here so one could sweep out the junk then rejuvenate the stem cells afterwards.
Bioviva Sciences as Josh is aware will be testing hTERT therapy in Humans this year using AAV delivery, their treatment however is not transient so we are looking at permanent hTERT up-regulation, I am wondering what the effects of this will be and if this permanent activation is a wise idea.
Also in agreement with Fred about the immune system being key to long life, if the Telomeres are restored in these cells is it logical to expect the immune system to up its game and resume a more youthful function? This has been seen to be the case in animal studies.
Are telomeres the magic bullet to stop aging? I doubt nature would put all her eggs in one basket but I have no doubt Telomeres are a key player in aging and dysfunction, the Telomere positioning effect I think is the most potent function they have and this causes Epigenetic drift which leads to aging.
Steve, out of curiosity, what are your thoughts about L-Carnosine? It’s not going to be miraculous by any chance, but it might buy a couple of years. It also seems to have some other health benefits.
I cannot prove this, but I suspect that the study is flawed in that it is the senescent cells that are the the problem. Shorter telomeres = more likelihood of forming more of these cells? So on average, people with longer telomeres would be more likely to live longer, because on average they would have fewer senescent cells at a given age? Does this sound like a reasonably hypothesis?
What might be worth looking for:
– telomere length on average vs number of senescent cells
– senescent cells vs life expectancy
It’d be interesting especially to see those outliers – how long do the ones with 10000+ base pairs at say, 60+ live?
That should give us a better idea of telomere length and life expectancy.
@Chris L-Carnosine I am not sure about but assume it falls into the realms of supplements/vitamins and nutraceuticals. To be honest I do not put much stead in supplements personally and believe the only way we can achieve radical life extension is via direct intervention eg, gene therapy, Stem Cell replenishment and Senlyotics.
However I am merely a layman and not a scientist but I don’t think we will move the needle much until we start using the needle literally and directly intervene in aging.
HI Steve funny you should mention the global positioning effect. I made a remark 2 years ago to Dr Fossel saying this whole thing must be epigenetic in the main. He stated, “Why would you think that?” and thus went the discussion. I alluded to the DDR both near and far in one of my above posts.
I didn’t realize Bioviva was that far along in their trials. That is good news but I do hope they complete both small and large animal studies. I understand Fossel and Blasco are also collaborating in similar but separate fashion with an AAV based platform with a planned FDA route. Maria’s initial delivery was bolus with a 1 year dilution degradation time. I like you I think would be concerned about constituitive telomerase activation especially in people who already have a compromised genome. Then again these are 100% fatal neurodegenerative diseases so the risk/benefit possibility is seemingly higher. As to supplements, most serious scientists would agree they are not going to cure any diseases and that there is a paucity of well controlled studies for obvious reasons including costs. I have no personal vested interest in TA-65 or other supplements but I would be a little lighter than calling it snake oil. Blasco Fossel, Andrews, Cal Harley and others have publically stated they are on it, and Blasco and Harley have been involved in studies that investigated its uses with positive outcomes. Those studies are easy to criticize because of design and limited size but they were at least attempts to look at the effects in cohorts. In addition Harley presented some compelling invitro and in vivo data on CMV patients and HIV patients with significant viral load values at AACR Telomerase Meeting
2007.12.07. Sadly they are the property of Geron so if you were not there or do not know him personally you are not likely to have access.
I am reasonably sure that Mr Kekich who is on Bioviva’s advisory board takes some sort of supplemental telomerase activator as well given his association with people who represent those companies. I could be wrong about that however.Similarly I have through my clinical experiences seen serial improvements in PMC MTL and % of short telomeres in every patient we had the chance to test for other reasons than to substantiate the efficacy of any supplement. In addition we have seen someone startling reductions in bone marrow fat and increased cellularity in some of our older patients on that product which while not randomized controlled, do not seem to be due to any other alterations in therapy or life style changes since these people are not particularly paragons of health. While these prove nothing they have tempered my attitude towards the possibility that snake oil may have some potential regenerative benefits at least in the immune compartment. If in your clinical experience, you have had very different results I would be interested in hearing them. Thanks! Fred
TA65 and product B etc… may sightly mitigate accelerated aging but personally I don’t think they do a great deal, same goes for many of these expensive supplements, they are just not going to add much in terms of lifespan IMO and the way forward is direct intervention.
Telomere positioning effect (Wright and Shay) and TPE over long distance I believe is a huge driver of epigenetic drift leading to dysfunction and aging. As telomeres shrink they cease silencing genes and we know that many more genes are expressed as we age compared to young. This suggests to me that without a doubt the primary function of Telomeres is gene regulation rather than a simple mitotic clock. If we target telomeres I firmly believe we will see large increases in life span and restoration of function.
Bill Williams has said he doesn’t know why we see rejuvenation from telomere extension(singularity 1-1 interview) but my bet would be TPE silencing aging gene expression, improved cell stability and likely Stem cells that were dormant returning to work. This last effect is also seen in parabiosis where blood factors do similar, this again could be factoral influence on Telomeres. Odd thing is few studies have measured changes to Telomeres when young factors are circulated in old systems. I linked a study that did measure this eariler and it showed that factors can and do increase Telomeres in primary hematopoietic cells:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2745844/
This suggests to me that Telomere length is almost certainly a key player in aging and that the TPE/gene regulation is the primary function with replicative clock being secondary. I would be interested on your thoughts concerning the above study and if we can expect similar effects from factors in other cells types.
You mention Dr Blasco, whilst I have a great deal of respect for her work I take her comments about TA65 with a pinch of salt, bearing in mind she has a stake in a Telomere measuring company. Not to say her work with AAV gene therapy is not impressive and I am hoping she gets her work into the human model soon. We know enough now to start taking the first steps into the human model and there is only so much sitting on the fence we can do, we have known about this for decades.
Speaking of BioViva they are in H+ again and are speaking at BRINK next month. They are very ambitious and look set to bring their technology to the human model very soon.
http://hplusmagazine.com/2015/05/06/bioviva-gene-therapy-to-treat-aging-and-beyond/#.VUrYltAuDOk.facebook
Regards clinical experience unfortunately I have none I am just a research rat who reads a lot of papers, speaks with researchers and tries his best to form an opinion on how aging can be tackled. I see gene therapies as a very good step towards mitigating dysregulaton and ultimately capable of halting aging decline in its tracks. We don’t know all the pathways yet but we have enough knowledge IMO to start working on therapies now, its the only way we are going to move forward.
Steve, Gabor Chris and everybody! I promised myself and all of you I would not “dominate this blog” It’s Josh’s show and I appreciate it as well as his patience but, he’s moved on LOL. I do have a few general comments to end my participation and wish all of you the absolute best in your quests for the truth in longevity. No doubt we are getting closer!
Overall there is a gigantic opportunity for many topics to be examined in the “light” of telomere length. I am so pleased to see the slow and steady increase both from the lay public and the science world. That said, until there is a pot of gold at the end of the rainbow for the pharmaceutical industry that is attached to low risk it is unlikely that things will happen much faster than now. At a lecture I gave at A4M in 2009 I said that the keys to aging would be found by privately funded industrial scientists- then the pharmaceutical industry would swoop in and buy it all up .I still think that will happen but in the meantime the funding has to come from private industry which means a ton of very useful and important studies will remain undone. I am certainly hopeful that BioViva has success as I am for the Blasco- Fossel collaboration. I predict the telomerase inhibitors will be of limited or no value ultimately in cancer but might be of value in “pre cancerous” disorders like mylodysplasia. Time will tell.
For telomerase activation I am personally in favor of the pharmaceutical approach which like most drugs will start with a nutraceutcial that will then be changed and made more potent for patent purposes- 95% of the drugs on the market started this way in spite of what we are led to believe. I think the “on- off” nature of this will be safer than a consituitive telomerase activation with genes or a virus, unless or until a genetic off on switch is constructed. Again drugs are a candidate for this- DePinho used a common standard in tamoxifen or the Sleeping Beauty transposon etc.
As to supplements in general or anything for that matter that supposedly turns on telomerase- to me the only thing that matters is the decrease in critically short telomeres with the eventual shift in the median to “longer”. Sadly most of the telomere measuring companies have not done well on the market. Probably and idea that is too soon in most cases. Having seen the vagaries of real time TRAP assays I am not in favor of them as a measure of efficacy for defining what works and what does not.
Finally Dr Fossel sent me a pre pub copy of his book. It is wonderful and I encourage you to pre order it on Amazon! That is a shameless plug but an honest one! All the Best to you folks!
In order for DNA polymerase to duplicate a chromosome strand, it has to attach to the end of the telemere on the end of the strand. In doing so it covers up some DNA bases, which therefore do not get duplicated. As the process continues for enough cellular divisions, eventually there is no more telemere left. One can see that when the DNA polymerase attaches to a chromosome strand without a telemere tail, then the DNA is going to cover up some actual genetic material, maybe even part of a gene. Naturally this cannot be good. Nevertheless, it seems that the duplication should work we;; until the telemeres are used up. This would result in short telemeres working great, until they were all used up; causing a sudden failure.
Fortunately for bacteria and mitochondria, they have circular chromosomes which do not shorten every time they are duplicated.
I’m hoping someone reading this can help me with a fact I’ve lost track of. It’s for an article on telomeres and aging.
There have been a number of studies that seem to show how certain lifestyle changes can increase the length of our telomeres. 10% seems to be the amount most often quoted. But I can’t seem to locate in any of the articles I have what this equates to in number of years. Does anyone know? (I also need a link to the relevant article).
Thank you for your help!
Thank you for your article. I just got my Telomere result. I’m 41 and my Telomere score is 5.6. Reading all the comments bellow made me question why did we allowed Telomere testing for commercial purchase when there is still so much to be explained. It seems to me that the research has just began and there are many questions left unanswered. Delivering results to general public in mail without any explanation and support can have horrible effects. It makes me wonder if the entire endevour is more money driven than humane in nature. I am still in shock and that my Telomeres are so short..I think reading results just got them even shorter. Does not seem ethical to me. Think how we handle HIV testing? Perhaps distributing Telomere test should be conducted in the same manner. If scientists still cant understand most of it, how in the world are regular folks to understand the results. To me personally this was like receiving death penalty!
It appears that the two dominant theories of aging are mitochondrial dysfunction and telomere length deterioration.
While TA-65, as a weak telomerase activator, may mostly solve for the telomere cause of aging, it may not necessarily solve for mitochondrial dysfunction.
I was diagnosed with Parkinson’s three years ago. Through a targeted supplement strategy, addressing the top two theories detailing the cause of PD, plus the three secondary causes, I have successfully reversed my PD symptoms by 1-2 years prior to diagnosis. More importantly, I have not progressed with my (now rare) PD symptoms in nearly 3 years.
I already take most of the supplements recommended to combat aging (and then some) as part of the PD protocol.
But now that I have solved for PD, I am very interested in solving for aging.
What would be very interesting is embarking on path that attempts to address both dominant theories of aging, instead of just one.
I just started taking TA-65 (250 units morning/bedtime) for telomerase activation and 15mg of MitoQ and 500mg of NAD+ precursor for mitochondrial restoration.
The following I have been taking for about 2.5 years:
I also already take a large dose of Omega 3 a few times a day
Curcumin
Resveratrol
Complete Amino Acid Profile
ZMA
PQQ
Grape Seed Extract
Ubiquinol
Alpha Lipoic Acid
Triphala
Pyconogenol
L-Tyrosine
EGCg
Sunflower Lecithin
Garlic Oil
Fiber
Acetyl-L-Carnitine
MK-7
7 Keto
5-HTP
Digestive Enzymes
Calcium/Magnesium
Probitotics
Astaxanathin
Vit D3
Prostate Support
Acetyle-L-Carnosine
Choline/Inositol
TUDCA
NAC
Vit A
Uridine
Collagen
L-Glutamine
IGF-1
Pyruvate
MCT Oil
L-Citruline
BCAAs
CLA
Beta Alanine
Multi
Most of those are for PD support, some for bodybuilding.
I am looking forward to the anti-aging components I am adding. I took pictures and I am awaiting the results of a telomere test.
Jon – I wish it were so simple. Telomere length and mitochondrial dysfunction are both aspects of aging, but the root cause of aging is epigenetics, and the ultimate solution will be epigenetic reprogramming. In other posts on this blog I have described this point of view in detail. Most other scientists in the field are less optimistic than I am, and think that age reversal will require re-engineering the body to repair damage at the molecular level, and there are many who believe that age reversal is impossible in theory.
About supplements: Like you, I take a long list of supplements. Many of these have been studied singly, but we know nothing about how they interact. This is a crucial point. We know that they interact strongly, and that most of the interactions are negative–this is not questioned. What we don’t know is what combination of supplements can synergize positively, and what the benefit can be from getting the combination right.
– JJM
A candidate signaling system for synchronizing aging across the body is the the Primo vascular system, a network of tiny vessels which go everywhere, penetrating and traversing the arteriovenous and lymphatic systems and organs. These vessels are so small and transparent that they remain unobserved except with very specific staining developed in Korea. They are largely unknown elsewhere. In cross section they contain a cluster of even smaller vessels, reminiscent of a cluster of wires in an electrical conduit. The Primo system appears to have nodes at acupuncture points.